DIMETHICONE COPOLYOL 93 CH 3 CH 3 CH 3 CH 3 CH 3---Si---(-O---Si---)a---(-O--Si---)b--O--Si--CH 3 CH 3 (CH2) 3 CH 3 CH 3 I O-(CH2CH20)x-H It must be clearly understood that the polymer structures shown above are equilibrium mixtures having a nominal or average structure. This is not unlike ethoxylated alcohols that are also oligomeric mixtures of different ethoxylated isomers (4). NOMENCLATURE In order to better understand the polymer chemistry, a shorthand has been developed that is more enlightening to the chemist than the name DMC. Developed in the 1940s by Dow Corning scientists, the nomenclature is based upon the type of groups present in the molecule. CH 3 I "M unit" is monosubstituted (one oxygen atom shared by the silicon) -O-Si-CH3 I CH• CH• I "D unit" is disubstituted (two oxygen atoms shared by the silicon) -O-Si-O- I CH• O I "T unit" is trisubstituted (three oxygen atoms shared by the silicon) -O-Si-O- I CH• O I "Q unit" is tetrasubstituted (four oxygen atoms shared by the silicon) -O-Si-O- I o If organofunctional groups other than carbon are introduced, the group is given an asterisk (*), which is added to its designation. "M* unit" is monosubstituted (two oxygen atoms shared by the silicon) with organofunctionality CH 3 I -O-Si-CH 3 I R
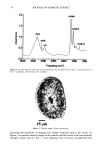
94 JOURNAL OF COSMETIC SCIENCE "D* unit" is disubstituted (two oxygen atoms shared by the silicon) with organofunctionality "T* unit" is trisubstituted (three oxygen atoms shared by the silicon) with organofunctionality CH 3 I -O-Si-O- I R O I -O-Si-O- I R There is no "Q* unit" since there is no possibility of functional groups. Thus, for example, the structure for MD2D3*M is: CH 3 CH 3 CH 3 CH 3 CH3-Si--(-O--Si--)2--(O--Si)3--O--Si--CH 3 CH 3 CH 3 (CH2) 3 CH 3 I O-(CH2CH20)x-(CH2CH(CH3)O)yH EXPERIMENTAL PROCEDURES In order to evaluate the properties of DMC polymers, the structures shown in Table I were synthesized via equilibration (2). The equilibration synthesis was conducted by blending two DMCs of differing molecu- lar weights at various ratios and reacting in the presence of caustic potash at 160øC for four hours. The percent polyoxyethylene group was kept constant. Physical properties were measured at room temperature, unless otherwise indicated, for the products made. These included solubility, spreading, emulsification, cloud point (per ASTM method D 2024), Draves wetting (per ASTM D 2281), foaming (per ASTM D 1173), surface tension/critical micelle concentration (cmc, using a Wilhelmy plate), and Draize primary ocular irritation (5). Table I Compounds Studied Calculated No. D*/No. D Adopted molecular weight units a designation b E.M.W. c 607 1.0/0 MD*M 607 808 1.3/0.9 MD*DM 621 1108 1.8/2.3 MD2*D2M 633 1610 2.5/4.5 MD3*D5M 644 2111 3.0/6.8 MD3*D7M 704 2412 3.6/8.1 MD4*D8 M 670 D* is -(CH2)3-O-(CH2CH20)7-H. No. D* and D units calculated from stoichiometry. No. D* and D units rounded off for simplicity. EMW = molecular weight divided by number of D* units (rounded to the nearest whole number).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)