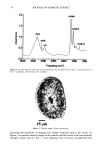
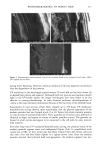
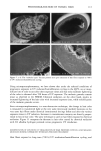
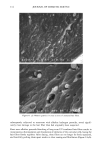
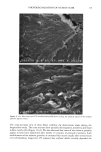
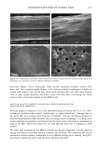
110 JOURNAL OF COSMETIC SCIENCE E 0.5 0.3 0.1 0.0 I I I I I I I 0 100 200 300 400 500 600 700 UV Irradiation (h) Figure 4. Progressive thinning and fusion of the surface cuticle cells as a function of exposure time to UV irradiation. layer of the cortex into one rigid unit causes brittle failure circumferentially and dis- places the stress of extension to those regions of the hair fiber that are still untouched by this progressive fusion. Multiple, successive fractures develop at individual sites along the cortical cell boundaries, then change direction and travel radially across individual cortical cells, and this pattern continues until it tapers off towards the core of the fiber, thereby creating what we call the "cathedral spire" fracture pattern (Figure 7a). The opposite, corresponding site of the "cathedral spire" fracture shows a hollow opening (Figure 7b), surrounded by a firmly fused wall. Higher magnification shows that this wall consists of a firmly fused cuticular sheath and possibly also the outer layer of cortical cells (Figure 7c,d). Some cuticular regions, preferentially those on the side of the fiber oriented towards the damaging light source, have become indistinguishable, rigid, and very brittle. The "cathedral spire" fracture pattern clearly shows primary levels of photodegradation in the fiber periphery (cuticular sheath) and a drop-off to lesser levels of degradation in the fiber interior. PHOTOCHEMICAL VERSUS CHEMICAL OXIDATION Differences between chemical and photochemical oxidation of hair proteins and melanins have been widely discussed in the literature. Robbins (9) reports that both chemical and photochemical oxidation attack both the hair pigments and proteins, and within the proteins, primarily the amino acid cystine. Up to 25 % of the disulfide bonds in human hair are degraded by "normal" bleaching, and 45 % of the disulfide bonds may be broken
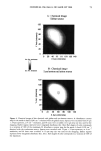
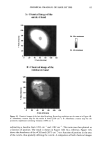
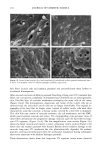
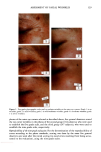
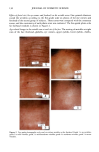
PHOTODEGRADATION OF HUMAN HAIR 111 TRI { - . Figure 5. Representative cross-sectional views of the cuticular sheath of an untreated (al,a2) and a 300-h UV-exposed (b) hair fiber. during severe bleaching. However, chemical oxidation of the hair pigments occurs faster than the degradation of the proteins. UV irradiation in the wavelength region between 254 and 400 nm has been shown (9) to degrade hair protein and pigment. Although both hair proteins and pigments absorb light in the UV/visible region, the longer wavelengths have been found to be less effective in causing photodamage. As with chemical oxidation, photodegradation of cystine is the most extensive phenomenon because of the reactivity of the disulfide bond. Examination of cross sections of hair fibers exposed up to 700 hours UV-irradiation/ humidification cycling showed, quite surprisingly, that the physical appearance of the melanin granules had not changed much, if at all. Figure 8a,b shows melanin granules in cross sections of untreated hair fibers. These granules are of various sizes, spherical or elliptical in shape, and appear to consist of smaller granular entities. The granules are housed in small cavities and appear to be connected to the cell walls by some intercel- lular material. Even after long-term UV-irradiation/humidification cycling, the physical nature of the melanin granules appears intact and undegraded (Figure 9a,b). In unpublished work carried out at TRI, we have shown that hair fibers retained their dark brown color and that only a few had been faded slightly to a lighter brown color. Since the melanin granules retained their physical bulk and appearance, the melanin pigment was pro-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)