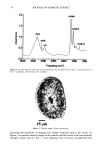
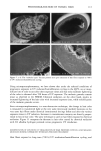
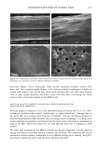
DIMETHICONE COPOLYOL 101 40 .u 20• lO • .=• o 500 1000 1500 2000 2500 • Irritation Wetting .... O/W Stability - - - Non-polar Solubility Molecular Weight Figure 6. Correlation of primary ocular irritation and physical properties. efficient compromises when one desires a low irritation potential. In general, the trends and observations made include: ß Solubility in polar media seems to relate to the amount of polyoxyethylene group. ß Products with higher molecular weight had better dispersibility in nonpolar oils. ß The cloud point is related to the amount of polyoxyethylene group in the molecule and is rather independent of the silicone portion of the molecule. ß The molecules studied spread better than water but cannot be considered superspread- ers. ß The lower-molecular-weight materials have faster wetting times, higher initial foam, and are more stable than the emulsion of oil in water. ß The higher the molecular weight the lower the ocular irritation. REFERENCES (1) R. M. Hill, "Siloxane Surfactants," in Specialty Surfactants, I. D. B. Robb, Ed. (Blackie, London, 1997), p. 143. (2) I. Schlachter and G. Feldmann-Krane, in Novel Surfactants, K. Holmberg, Ed., Surfactant Science Series, Vol. 74 (Marcel Dekker, New York, 1998), p. 201. (3) R. M. Hill, Silicone Surfactants, Surfactant Science Series, Vol. 86 (Marcel Dekker, New York, 1999). (4) A. L. Smith, Ed., The Analytical Chemistry of Silicones, Chemical Analysis Series, Vol. 112 (John Wiley & Sons, New York, 1991). (5) J. H. Draize, "Dermal Toxicity," in Appraisal of the Saj•ty of Chemicals in Food, Drug and Cosmetics (The Association of Food and Drug Officials of the United States, 1954), pp. 49-51. (6) S.C. Vick, Soap/Cosmet./Chem. Spec., 36 (May 1984). (7) K. P. Ananthapadmanabham, E. E. Goddard, and P. Chandar, Colloids Surfaces, 44, 281 (1990). (8) T. Svitova, H. Hoffmann, and R. M. Hill, Lungmuir, 12, 1712 (1996). (9) T. Stoebe, Z. Lin, R. M. Hill, M.D. Ward, and H. T. Davis, Lungmuir, 12, 337 (1996). (10) N.E. Prieto, W. Lilienthal, and P. L. Tortorici,J. Am. Oil Chem. Soc., 73, 9 (1996). (11) M.J. Rosen, J. Am. Oil Chem. Soc., 49, 293 (1996).
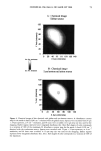
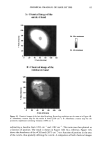
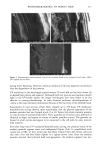
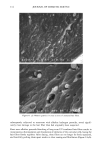
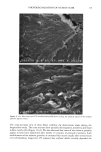
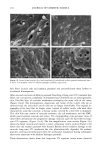
j. Cosmet. sci., 51, 103-125 (March/April 2000) Photodegradation of human hair: An SEM study SIGRID B. RUETSCH, Y. KAMATH, and H.-D. WEIGMANN, TRI/Princeton, P.O. Box 625, Princeton, NJ 08542. Accepted for publication January 3 I, 2000. Synopsis This study uses field emission scanning electron microscopy (FESEM) to monitor the effects of UV irra- diation on the physical nature of hair fibers. Long-term UV irradiation/humidification cycling causes thinning and fusion of the surface cuticle cell, as well as fusion of the cuticular sheath into a solid, rigid, and brittle unit. While intercellular cohesion within the cuticular sheath is high, possibly due to crosslink- ing of the proteins in the intra- and intercellular domains, the cells themselves are brittle. A newly observed fracture pattern of long-term UV-exposed fibers suggests fusion of the regions attacked most severely by UV light into one rigid and brittle mass, incapable of extension due to loss of all original elastic properties. Unlike chemical oxidation, which results in partial dissolution (1 h H202) and then complete solubilization (4 h H202) of the melanin granules, photochemical oxidation produces entirely different results. Even after long-term UV irradiation/humidification (95% RH) cycling, the melanin granules appear physically intact. Loss of color does not occur as long as the melanin granules are intact. The severity of photodegradation during UV irradiation/humidification cycling becomes apparent upon brief (seconds) contact of these fibers with alkaline hydrogen peroxide. Such contact results in instantaneous disintegration of the components within the cuticle cells. Formation of sac-like structures (AllwiSrden sacs) occurs due to osmotic pressure within seconds of exposure to alkaline hydrogen peroxide caused by pho- tochemically degraded proteins within the surface cuticle cells. The cells swell until they burst and their contents drain, leaving behind cuticular membranes, which may detach or fuse to the fiber surface. UV irradiation has also severely photodegraded the melanin granules and preconditioned them for accelerated solubilization upon contact of the fibers with alkaline hydrogen peroxide. The effects of both relative humidity and spectral energy distribution on the photochemical oxidation of the hair fiber are studied. Results obtained at various relative humidities in two different fading units, namely, the QUV Accelerated Weathering Tester and the Atlas Weather-Ometer © ("AW") are compared. Scale thinning and fusion observed during UV/humidification cycling are greatly reduced with exposure at low humidities without humidification cycles. Upon post-treatment with water, fibers irradiated at a constant 10% RH in the QUV show scale thinning and fusion similar to that of fibers exposed to UV/humidification cycling. This indicates that photodegradation occurs at low humidity as well. Fibers exposed at constant 20%, 50%, and 70% humidity in the "AW" show only moderate scale thinning, even after post-treatment with water. The total solar spectrum used in the "AW" apparently causes less severe photodegradation of the proteins than the UV light of the QUV. INTRODUCTION When exposed to sunlight, hair is known to undergo changes in morphological, chemi- cal, and mechanical characteristics (1-4). The lower wavelength range of the UV com- 103
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)