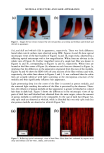
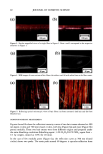
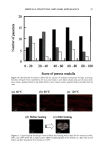
j. Cosmet. Sci., 53, 81-87 (March/April 2002) Comparison of the moisturization efficacy of two uaoinal moisturizers: Pectin versus polycarbophil technolooies MICHAEL CASWELL and MICHAEL KANE, C.B. Fleet Company, Inc., 4615 Murray Place, Lynchburg, VA 24502 (M.C.), and 525 Union Boulevard, Totowa, NJ 07512 (M.K.). Accepted j•r publication December 31, 2001. Synopsis This study was designed to compare the vaginal deposition and moisturization of two vaginal moisturizers, Summer's Eve © (SE), based on pectin, and Replens © (Rp), based on polycarbophil, in a double-blind crossover study design. Fifty-one female patients were each randomly assigned to one of two treatment groups. After a one-week washout period, the products were used for two weeks. After another one-week washout period, product assignments were switched. Colposcopy examinations were performed at the beginning and at the end of each product use. Of the forty-seven patients completing the study, 41 (87%) were found to have no vaginal residue after SE vaginal moisturizer, while only 25 (53%) were found to have no vaginal residue after using Rp vaginal moisturizer. No difference in relief of vaginal dryness or in product acceptance was found between the two products. This study shows that the use of SE vaginal moisturizer, based on pectin, resulted in significantly less vaginal residue compared to Rp vaginal moisturizer, based on polycarbophil, and in comparable relief of vaginal dryness. These results strongly suggest that bioadhesion is not important in vaginal moisturizers. INTRODUCTION Common problems associated with menopause are vaginal dryness with associated irri- tation and itching (1). A common therapy for vaginal dryness resulting from menopause is estrogen replacement therapy. However, nonhormonal therapies have been shown to be as efficacious in relieving vaginal dryness as hormonal therapies. For example, studies have shown Replens © (Rp), a non-hormonal vaginal gel composed with a polycarbophil bioadhesive, to be as effective as a hormonal cream in the relief of vaginal dryness (2,3). Rp adheres to the epithelial cells lining the vaginal walls and is only detached upon desquamation of the outer layer of cells. Kelly (4) reported that Rp "offers relief that lasts Address all correspon'dence to Michael Caswell. 81
82 JOURNAL OF COSMETIC SCIENCE for days at a time." Indeed, Rp is so adherent that it has been used as a vaginal drug delivery system (5). To date, only one piece of evidence supports the hypothesis that bioadherence of the carbomer-polycarbophil is not important in the relief of vaginal dryness. While Bach- man et aL (6) report that this bioadhesion holds water in place on the vaginal epithelium, they also report as little as 7.8% vaginal residue in women using Rp, after five con- secutive days of use. This suggests that bioadhesion of polycarbophil is not necessary for relief of vaginal dryness. A new product, Summer's Eve © (SE) vaginal moisturizer, avoids the use of bioadhesives by using pectin while providing natural moisturizers to relieve vaginal dryness. This clinical study was designed to compare the patient-perceived vaginal moisturization properties of Rp to those of SE and to evaluate the amount of vaginal residue resulting from the use of each product in a double-blind crossover study. MATERIALS AND METHODS PATIENTS Fifty-one postmenopausal patients aged 41-67 (mean age 52) who met all study inclu- sion and exclusion criteria (Table I) were admitted into the study. The study began on April 1, 1999, and ended on May 14, 1999. The two products tested in this study are described in the literature. Each patient was randomly allocated to a treatment group. Each group went through a one-week washout period, during which time no sexual lubricant/moisturizer was used. Group A used SE vaginal moisturizer every other day for two weeks, avoided the use of any lubricant for one week, and then used Rp every other day for two weeks. Group B used Rp every other day for two weeks, avoided the use of any lubricant for one week, and then used SE vaginal moisturizer every other day for two weeks. The use of each Table I Inclusion and Exclusion Criteria Inclusion criteria Exclusion criteria Healthy females, aged 40 years and older who report vaginal dryness due to perimenopause, menopause, or oophorectomies Females who normally have sexual intercourse at least once per week Absence of history or visible evidence of chronic skin disease or regional infection Dependability and intelligence to follow directions Willingness to cooperate No history of sensitivity to similar formulations Completion of medical history form Understanding and execution of the informed consent agreement Females who have any disease or condition that, in the opinion of the gynecologist, could affect the test results Females using hormonal replacement therapy Females with a history of genital herpes, recurrent vaginal infections, or urinary bladder infections Females using medications that might influence the test results Females with a history of allergy or hypersensitivity to any female hygiene product
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)