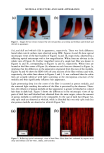
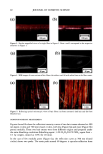
2001 ANNUAL SCIENTIFIC MEETING 131 Coacervate identification was made by separating the hazy material after centrifugation during 1 hour at 3,000 rpm. At the end of the centrifugation process the dispersed coacervates separated into a clear and transparent gel layer at the bottom of the centrifugation tube. Fig. 1 shows a typical microscopic image of coacervates dispersed in the shampoo dilution before centrifugation. In order to analyze the behavior of coacervates in foams, the same hazy shampoo dilution was stirred in a beaker until a dense foam was produced. A microscopic image of the foam surface showing the presence of coacervates dispersed in the inter-lamellar liquid of the foam can be seen in Fig. 2. This observation clearly indicates that coacervates formed during shampoo dilution persist during the foaming process. Further dilution foam analysis of model shampoos indicated that coacervates partition between the lamella walls ("crust") and lamellar liquid ("core") of the foam (3). For instance, when foams made of shampoos at different dilutions were poured into a separatory funnel and allowed to stand 5 minutes, the foam separated into two main components, namely: a drained liquid at the bottom of the funnel and a dry foam at the top of it. The drained liquid of foams made with shampoos at low dilutions was found to be hazy and to contain dispersed coacervates. It was also seen that as the foam dilution ratio increased the drained liquid become clear and transparent. At higher foam dilutions the drained liquid turned hazy again. These observations indicate that coacervates partitioned into the liquid "core" of the foam lamella change from dispersible to non- dispersible, and then again to dispersible with the dilution ratio. The interaction of silicone droplets with coacervates was affected by the observed variations in coacervate dispersability. For instance, at low shampoo dilutions when the coacervates were dispersible, coacervate and silicone droplets behaved as independent dispersible entities (see Fig. 3). This observation indicates that at low dilutions the interaction between coacervates and silicone droplets is practically non-existent. In contrast, at dilution regions where the coacervates became non-dispersible, it was found that silicone droplets and coacervates flocculated forming complex aggregates (see Fig. 4). At higher dilutions the silicone/coacervate flocs became weaker and started to separate. Fig. 1) Microscopic image of 1 to 7 dilution of shampoo 15 % SLES-2/0.5 % JR-30M/3% CAPB showing coacervate gel particles dispersed in shampoo solution 37X
132 JOURNAL OF COSMETIC SCIENCE Fig. 2) Microscopic image of foam surface made with shampoo 15 % SLES-2 0.5 % JR-30M/3.0 % CAPB showing coacervates in lamella foam (dilution I to 7) I Coacervates Fig. 3 Microscopic image of silicone droplets and coacervates showing in&pendent dispersion Shampoo: 15 % SLES/0.5 % JR-30M 3.0 % CAPB Silicone oil GE SF-96-50 (dilution I to 3) 37X Fig. 4) Microscopic image of silicone droplets and coacervates showing flocculation and formation of aggregates. (Shampoo: 15 % SLES/0.5 % JR-30M 3.0 % CAPB Silicone oil GE SF-96-50) (dilution I to 7) 37X REFERENCES 1) J.V. Gruber, B.R. Lamoreaux, N. Joshi, L.Moral, "Influence of Cationic Polysacchafides on Polydimethylsilixane Deposition onto Keratin Surfaces from a Surfactant Emulsified System", pp. 127-135, Col. and Surf. B: Biointerfaces, 19 (2000) 2) R.T. Jones, and C.A. Brown, "The Behavior of Cationic Cellulose Derivatives Containing Fatty Quat Groups", Int. J. of Cosm. Chem., 38, pp. 233-246 (1987)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)