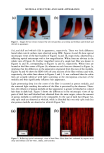
114 JOURNAL OF COSMETIC SCIENCE 3000 2250 - 1500 - 750 - 1 4 7 10 -200 I I 0.0 7.5 15.0 22.5 13 Retention time (min) 35.0 3000 2250 - 1500 - 750 - -200 0.0 b 4 14 13 I I I 7.5 15.0 22.5 35.0 Retention time (min) Figure 4. Chromatographic separations of two commercial formulations after spiking and matrix extrac- tion. a: "dark brown" shade shampoo. b: "dark blonde" shade shampoo. Peaks: (1)p-phenylenediamine (2) hydroquinone (4) m-aminophenol (5) toluene-2,5-diamine sulfate (6) 2,4-diaminophenoxyethanol (7) resorcinol (9) toluene-2,4-diarnine (listed in Annex II, deliberately spiked) (10) N,N'-bis-(2- hydroxyethyl)-p-phenylenediamine sulfate (13) hydroxypropyl-bis-(N-hydroxyethyl-p-phenylenediamine) HC1 (14) ot-naphtol and (M) matrix compound. See text tbr experimental data and acronyms.
OXIDATIVE HAIR DYES 115 Table IV Peak Asymmetry Factor (s) and Capacity Factor (k', dead time = 2.50 min) for the Nine Hair Dye Peaks in the Two Spiked Formulations DBRS DBLS s k' s k' Peak RSD (%) RSD (%) RSD (%) RSD (%) p-Phenylenediamine 0.88 1.26 * * 2.07 0.43 Hydroquinone 1.14 1.78 * * 1.44 O.79 m-Aminophenol 1.09 2.31 1.04 2.27 1.63 0.81 4.76 1.08 Toluene-2,5-diamine sulfate 1.09 2.79 1.15 2.82 3.70 0.60 2.64 1.40 Resorcinol 1.08 3.23 1.08 3.07 1.65 0.80 1.68 1.16 2,4-Diami nophe noxye thanol 0.85 3.63 0.85 3.30 0.53 1.10 1.48 1.17 Toluene-2,4-diamine • * * 1.02 4.77 0.97 0.71 Hydroxypropyl-bis-(N- 1.35 9.57 1.15 9.50 hydroxyethyl-p-phenylenediamine) HC1 4.93 0.44 3.93 0.89 o•-Naphtol * * 1.05 11.38 2.78 0.08 * Not present in the formulation. a Listed in Annex II (banned compound), deliberately spiked. s and k' are given with the relative standard deviation RSD (%) calculated on ten consecutive injections. of concentrations could be separated with a good resolution comprised between R6, 7 -- 1.14 and R5, 6 = 1.55 (see peak labeling in Figure 4). The identification of the hair dye intermediates was performed as described previously. It has to be noted that in these conditions, the elution order is inverted for some compounds. Peaks 1, 2, 4, 5, 7, 6, 9, 13, and 14 are attributed to p-phenylenediamine, hydroquinone, m-aminophenol, tolu- ene-2,5-diamine sulfate, resorcinol, 2,4-diaminophenoxyethanol, toluene-2,4-diamine, hydroxypropyl-bis-(N-hydroxyethyl-p-phenylenediamine) HC1, and tx-naphtol, respec- tively. Peak 10 is attributed to an additional hair dye present in this formulation according to the ingredient labeling (N,N'-bis-(2-hydroxyethyl)-p-phenylenediamine sulfate). Peaks labeled M are shown to be due to remaining matrix compounds. One remark can be made for p-phenylenediamine in DBRS that shows a broad peak in these conditions (peak 1 in Figure 4a). It could be demonstrated that this shape is due to the fact that at this pH (5.9), both basic and amphoteric forms ofp-phenylenediamine are present, as the acidity constants experimentally determined were 2.46 and 6.04 (the values given by NIST being 2.97 and 6.3). The two forms are thus eluted at very close retention times, leading to an enlargement of the peak.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)