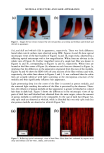
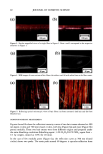
2001 ANNUAL SCIENTIFIC MEETING 149 CLINICAL EFFECTS OF EMOLLIENTS ON SKIN Joachim W. Fluhr, M.D. Department of Dermatology, VA Medical Center and University of California, San Francisco, CA Introduction Recent studies have shown that the use of an appropriate emollient for the treatment of spectfic skin disorders can have a significant impact on both the clinical outcome of treatment, and the extent of the subsequent relapse-free period. Emollients should not be regarded simply as a drug career or delivery system, but rather as an essential component of successful topical treatment. Specific effects ofemolhents Emollients exert hydrating, cooling and barrier repair effects on the skin, They influence the stratum comeum (S,C,) hydration, for which different mechanisms have been proposed: - The emollient exert a direct hydrating effect by liberating water from the formulation itself [1 ]. This effect of O/W-systems depends primarily on the water content of the formulation, with unbound water insuring water insuring the S.C. hydration [2]. In long-term applications of W/O but not of O/W emulsions revealed hydration of the S.C. [3]. - The occlusive effect of the formulation can inflt•ence S.C. hydration. especially in long-term applications e.g, petrolatum, for which the highest occlusive effect was detected [4]. W/O emulsions with low water content have occlusive effects similar to petrolatum, while W/O emulsions with high water content have occlusive properties similar to O/W emulsions [4]. Even O/W formulations with high water content exert an occlusion effect after the unbound water evaporates. In atopic dermatitis, an occlusive effect may enhance discomfort and induce itching response(s). The occlusive effects also may enhance drug penetration. - Highly hygroscopic compounds like glycerol, by absorbing water either from the emollient itself, from surface water, or from water of evaporation, then are able to increase S.C. hydration [5]. Recent studies in asebia mice could show the importance of sebaceous gland derived glycerol for S.C. hydration (unpublished data) (Fig. 1). - The formation of lipid bilayers from secreted lameliar bodies at the Stratum granulosungS.C. interface traps hygroscopic materials in the Comeocyte cytosol, with the comeOCyte lipid envelope acting like a 'dialysis membrane'. The cooling-effect of emollients can be attributed to the amount of evaporated water and/or alcohol in the emulsion, e.g, with lotions, hydrogels or O/W emulsions. However. relatively non-stable W/O emulsions, like cold creams, also can exert mild cooling-effect(s). Role of ph¾sioloqical lipids in emollient formulations The barrier function of the skin is mediated by intercellular bilayers in the S.C.. Cholesterol (25%), ceramides (40%), free fatty acids (20%) are key lipids in the formation of these bilayers [6]. These lipid classes have an approximately equimolar physiologic ratio. Following barrier disruption, epidermal cholesterol and fatty acid syntheses are immediately increased, while increased ceramide production is evident about 6 hours later [6-9]. The key lipids are delivered to the intercellular space of the S.C, as a mixture of precursors by the extrusion of lameIlar body content at the stratum granulosum - S.C. interface. Fusion of the secreted lameliar contents within the lower S.C. leading to continuous membrane sheets, ultimately form mature membrane bilayer structures. These membrane structural transformation correlates with changes in lipid composition: the polar lipid precursors are metabolized to more- nonpolar lipid products [6]. Topical applied physiologic lipids have distinct effects from those of nonphysiologic lipids, like petrolatum. Studies have shown that topical application of only one or two of the three physiologic lipids to disrupted skin impedes rather than facilitates barrier recovery [7]. If members of all key lipids are apphed together to barrier-disrupted skin, normalized rates of barrier repair are observed [10]. Further enhancement of barrier recovery is observed if the proportion of one of the fatty ac:ds (linoleic acid, palmitic acid or stearic acid) or either of the two other key species is augmented to three-fold i.e. consisting of fatty acid, ceramide, cholesterol, essential fatty acids in a 3:1:1:1 ratio [11]. Topically applied physiologic lipids not only are concentrated in the S.C. membrane domains, but also are delivered to the nucleated layers of the epidermis [7, 10] (Fig. 2). Depending on the composition of the lipid mixture, either normal or abnormal lameIlar bodies are formed, resulting in either normal or ab,c, rmal lameliar membrane umt structures in the S.C. intercellular spaces. The incorporation of applied physiolo•oic lipids into barrier lipids follows two pathways: 1) direct incorporation into S.C. membrane domains 2) lip•ds appear to traverse the intercellular route in the S.C., and ultimately get incorporated into lower stratum granulosum cells. The intercellular ilplds then appear able to enter the nucleated cells, incorporate into the appropriate lipid metabohc pathway(s), and ultimately utilize the lameliar body delivery system to reenter the intercellular membrane domains [10]. These studies support the hypothesis that the epidermis can internalize and process physiologic lipids..In contrast, nonphysiologic lipids like petrolatum appear to simply form a bulk hydrophobic phase in the S.C. intercelltfiar spaces to restore the barrier under similar condition [10]. Figure 1: Proposed sequence in Asebia-J skin: Decreased sebaceous gland-derived glycerol results in reduced SC hydration. Figure 2: Topically aoolied ohvsioloeic li•ids in lhe S.C. membrane and lhe nuclealed layers Lameliar body formallon in the granular cell. Topically applied physiologic lipiris enler lhe granular cell and reach sites of lameliar body formation (wilh permission from Dr. P.M, Elias)
150 JOURNAL OF COSMETIC SCIENCE Barrier protection and barrier recovery Commonly used barrier creams, which are either W/O emulsions or emollients with lipophilic character, are claimed to protect against hydrophilic irritants. On the other hand, barrier creams that are O/W emulsion systems, or that act like hydrophilic systems, are thought to protect against lipophilic irritants. Pre-exposure skin care includes the use of O/W- and W/O-emulsions, tannery substances, zinc oxide, talcum, perfluorpolyethers, chelating agents, and UV-protectors. However, cleansing products and post-exposure skin care are two other important components of skin protection. The post-exposure skin care is based on emollients, moisturizers, humectants and lipid-rich formulations. Cumulative stress tests with repetitive application of irritants appear to be the best conditions for approximating work conditions [12-14]. Emollients or vehicle alone often shows a significant improvement of the clinical skin conditions as well as the S.C. hydration [15]. These authors proposed that a strict distinction between skin care and skin protection products should not be maintained [15]. A recent study discussed whether claims could be made with respect to protective and preventive properties of topically applied body lotions and barrier creams [16]. Moisturizer-containing emollients prevent irritant skin reactions induced by detergents, and may also accelerate regeneration of barrier function in irritated skin [5, 17]. Emollients with moisturizing properties, usually contain either singly or in combination(s) humectants, such as urocanic acid, ammonia, lactic acid, pyrrolidone carboxylic acid, urea, citrate, glycerol, sorbitol and hydroxy acids. These agents belong to a group considered "natural moisturizing factors" (NMF), and/or moisturizers. Their properties include the increase of hydration and the enhancement of water binding capacity in the upper S.C., while reduced NMF can diminish water absorption capacity and may result in perturbation of comeodesmolysis leading to hyperkeratosis. Emollients in atopic dermatitis The utility of emollients in the treatment of atopic dermatitis is well recognized. In atopic dermatitis S.C. hydration and water binding capacity are reduced [18-19], and impaired barrier function is readily observed in both involved and uninvolved skin [18]. These patients also are more prone to develop an irritant contact dermatitis [20]. In atopic dermatitis S.C. the content of barrier lipids is reduced, most prominently that of ceramide I and ceramide 3 [21-22]. This reduction of ceramide levels may result from the over- action of the epidermis-unique enzyme, sphingomyelin deacylase [23-24]. Sebaceous gland activity also is reduced in these patients [25]. Moreover, in atopic dermatitis S.C., the lamellar bodies are incompletely extruded and organized. This, along with the altered S.C. lipid content, may explain the impaired barrier function in these patients. Thus, emollients for patients with atopic dermatitis should improve barrier function and hydration, should have protective properties and contain an antibacterial compound (e.g. triclosan) [26]. These demands are met by emollients showing W/O emulsions properties with a high water content, containing a moisturizer (e.g. glycerol) [5], and/or those based upon physiological lipid mixture, which are ceramide-dominant [10-11,27]. References 1. CW Blichmann, J Serup, A Winther. Acta Derm Venereo169:327-330, 1989. 2. M Loden. Acta Derm Venereo172:327-330, 1992. 3. JWFluhr, GVrzak, MGloor. ZHautkr73:210-214, 1997. 4. L Lehmann, M Gloor, S Schlierbach, W Gehring. Z Hautkr 73:585-590, 1997. 5. JW Fluhr, M Gloor, L Lehmann, S Lazzerini, F Distante, E Berardesca. Acta Derm Venereo179:418-421, 1999. 6. PM Elias, KR Feingold. Semin Dermatol 11:176-182, 1992. 7. M Mao-Qiang, PM Elias, KR Feingold. J Clin b•vest 92:791-798, 1993. 8. WM Holieran, MQ Man, WN Gao, GK Menon, PM Elias, KR Feingold. J Clin Invest 88:1338-1345, 1991. 9. KR Feingold, MQMan, GKMenon, SSCho, BEBrown, PMElias. JClinb•vest86:1738-1745, 1990 10. M Mao-Qiang, BE Brown, S Wu-Pong, KR Feingold, PM Elias. Arch Dermatol 131:809-816, 1995. 11. MM Man, KR Feingold, CR Thomfeldt, PM Elias. J Im,est Dermatol 106:1096-1101, 1996 12. AM Grunewald, M Gloor, W Gehring, P Kleesz. Co•tact Dermatitis 32:225-232, 1995. 13. W Wigger-Alberti, A Rougier, A Richard, P Elsner. Acta Derm Venereo178:270-273, 1998. 14. PJ Frosch, A Kurte, B Pilz. Contact Dermatitis 29:113-118, 1993. 15. U Bemdt, W Wigger-Alberti, B Gabard, P Elsnet. Contact Dermatitis 42:77-80, 2000. 16. K de Paepe, MP Derde, D Roseeuw, V Rogiers. Contact Dermatitis 42:227-234, 2000. 17. DW Ramsing, T Agner. Acta Derm Venereo177:335-337, 1997. 18. M Loden, H Olsson, T Axell, YW Linde. BrJDermatol 126:137-141, 1992. 19. E Berardesca, D Fideli, G Borroni, G Rabbiosi, H Maibach. Acta Derm Venereo170:400-404, 1990. 20. W Gehring, M Gloor, P Kleesz. Contact Dermatitis 39:8-13, 1998. 21. A Di Nardo, P Wertz, A Giannetti, S Seidenari. Acta Derm Venereo178:27-30, 1998. 22. G Imokawa, A Abe, K Jin, Y Higaki, M Kawashima, A Hidano. Jlnvest Dermato196:523-526, 1991. 23 J Hara, K Higuchi, R Okamoto, M Kawashima, G Imokawa. J Invest Dermatol 115:406-413, 2000. 24. K Higuchi, J Hara, R Okamoto, M Kawashima, G Imokawa. Biochem J 350:747-756, 2000. 25. H Wirth, M Gloor, D Stoika. Arch Dermatol Res 270:167-169, 1981. 26. W Gehring, T Forssman, G Jost, M Gloor. Akt Dermato122:28-31, 1996. 27. Chamlin SL, Frieden IJ, Fowler A, Williams M, Kao J, Sheu M, Elias PM. Arch Dermatol 137:1110-1112, 2001
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)