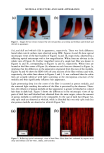
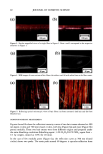
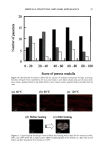
100 JOURNAL OF COSMETIC SCIENCE interaction with hair spatially characterized by infrared microspectroscopy using synchrotron radiation (Paper No. 33, 20th IFSCC Congress, Cannes, 1998). (7) J. L. Clement, A. LePareux, and P.F. Ceccaldi, The specificity of the ultrastructure of human hair medulla, J. Forensic Sci. Soc., 22, 396-398 (1982). (8) T. Yasuda, T. Sutoh, and N. Seta, Mouhatsu Kagaku Zusetsu 7th, Nihon Mouhatsu Kagaku Kyoukai (1996). (9) C. R. Robbins, Chemical and Physical Behavior of Human Hair, Third Edition, (1994). (10) Vlabo Valkovic, Human Hair (CRC Press, Boca Raton, FL, 1988), Vol. 1. (11) R.F. Stamm, M. L. Garcia, and J.J. Fuchs, The optical properties of human hair. I. Fundamental consideration and goniophotometer curves, J. Soc. Cosmet. Chem., 28, 571-599 (1977). (12) R. F. Stamm, M. L. Garcia, andJ. J. Fuchs, The optical properties of human hair. II. The luster of hair fiber, J. Soc. Cosmet. Chem., 28, 601-609 0977). (13) L.J. Wolfram and L. Albrecht, Chemical- and photo-bleaching of brown and red hair, J. Soc. Cosmet. Chem., 82, 179-191 (1987). (14) A. R. Haly and O. A. Swanepoel, The nature of birefringence, Textile Res. J., 31,966-972 (1961). (15) F. Wakui, Z. Shinjo, T. Ikeuchi, and N. Uchino, Study concerning the luster of human hair, J. Soc. Cosmet. Chem. Japan, 21, 156-161 (1987). (16) M. Glass, The diameter dependence of fiber medulladon and the medulladon weighting function. Textile Res. J., 70, 611-614 (2000).
j, Cosmet. Sci., 53, 101-119 (March/April 2002) Optimization and validation of an analytical procedure for the determination of oxidative hair dyes in commercial cosmetic formulations URSULA VINCENT, GUY BORDIN, and ADELA R. RODRIGUEZ, European Commission, Joint Research Centre, Institute for Reference Materials and Measurements, Retieseweg, B-2440 Geel, Belgium. Accepted for publication December 31, 2001. Synopsis A method has been developed and validated for the analysis of commonly used intermediates of oxidative hair dyes in commercial cosmetic formulations, including both liquid and cream forms, in dark and blonde shades. The commercial formulations are submitted to extraction by an organic solvent, and the resulting aqueous phase is analyzed by reverse-phase HPLC with a gradient elution and detection with DAD and/or ESI-MS-MS. A spectra library containing 200-400 nm spectra of the target substances and their HPLC retention times has been recorded for the identification. The quantification of the target substances is also performed after spiking of the commercial formulations, using an external calibration. The recoveries obtained are very good for all selected intermediates. The whole procedure has been found to be highly suitable for the identification and quantification of dye intermediates. Also implemented has been a database containing (a) the retention times, (b) the spectral, MS, and MS/MS characteristics of the intermediates, (c) acidity constant values of some intermediates of interest experimen- tally determined and compared to the available NIST values, (d) the chromatographic conditions used, (e) the behavior towards extraction of dye intermediates, and (f) matrix compounds. INTRODUCTION A high range and variety of cosmetic formulations for permanent hair dyeing is available on the market under several forms, from cream to liquid. In this paper, the liquid form will be referred to as a shampoo. All these formulations contain matrix compounds in which hair dye intermediates at different concentrations are embedded. Oxidative hair dyes are aromatic organic compounds, e.g., amines, phenols, and derivatives, while a broad range of products can be used as matrix compounds. The different shades are obtained not only by the use of mixtures of different hair dye intermediates but also by reciprocal adjustment of the relative concentrations of the same intermediates. Two opposite shades could thus be obtained by changing the relative proportions of the same oxidative hair dyes. The choice of commercializing a formulation as a shampoo or a lol
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)