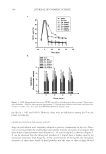
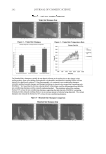
JOURNAL OF COSMETIC SCIENCE 82 CONCLUSIONS In vitro data suggest that different in vivo performances are expected for two dihydroxyac- etone (DHA)-containing formulations with similar concentrations of DHA and excipi- ents but different commercially available rheology modifi ers: one with a cationic polymer-based rheology modifi er (blend) [dimethylacrylamide/ethyltrimonium chloride methacrylate copolymer (and) propylene glycol dicaprylate/dicaprate (and) PPG-1 tride- ceth-6 (and) C10-11 isoparaffi n] and the other with a polyacrylamide-based rheology modifi er (blend) [polyacrylamide (and) C13-14 isoparaffi n (and) laureth-7]. These rheol- ogy modifi ers were used at 3% w/w (as supplied) and contained comparable concentra- tions of active polymers. In the followup in vivo studies, the test article with the cationic polymer-based rheology modifi er produced a more natural sunless tan, comparable to a desirable sun-induced tan, for all panelists the sunless tan was also more uniform and lasted longer compared with the sunless tan generated by the test article with the poly- acrylamide-based rheology modifi er. Panelists noticed a slightly less intense color devel- opment after application of X within the fi rst 24 hours, but the color of the tan was more even compared to that from K. These visual observations were confi rmed instrumentally: the average standard deviation of ΔE*ab for the panelists after 24 hours was 4.2 times higher for K than that for X. At 48 hours the ΔE*ab vs time trend was reversed, with slightly higher values for X than K. Apparently the fading of the sunless tan occurred at a slower rate with X than with K. Color associated with X was still visible with the “naked eye” after 120 hours for four panelists and for only one panelist with K. This indicates poten- tially better longevity of the tan generated by X compared to K, which may be attributed to the presence of the cationic polymer-based rheology modifi er (blend) in X. Overall, X produced a sunless tan color more consistent with the “natural universe of suntan tonal- ity” range, except for one panelist whose color was outside the range, but four other pan- elists had similar color responses and were well within the range. Figure 7. ΔC* and ΔL* values in vivo at 24 hours after application.
SUNLESS TANNING FORMULATIONS 83 As for the “natural universe of suntan tonality,” measurements associated with X were within this realm, indicating that the sunless tan generated by X is comparable to a desir- able natural sun-induced tan for all panelists. Application of K resulted in three panelists being outside the “natural universe of suntan tonality” range. Two of the panelists devel- oped a color that was more yellow than natural suntan, while another panelist seemed to have less red than natural suntan. In addition, one of the fi ve panelists was outside the “natural universe of suntan tonality” range and another panelist had a less developed tan that was on the borderline of the range. Overall, the tonality of the sunless tan generated by K was less “natural” since three out of fi ve panelists were outside the “natural universe of suntan tonality” range. The majority of panelists preferred the sunless tanning experi- ence associated with X. Their subjective perceptions were: X produces a more natural, more even, and longer-lasting tan that was initially less intense versus K, which gener- ated slightly higher ΔE*ab values in 24 hours, but faded away faster after 48 hours. An HPLC method for the analysis of DHA levels in sunless tanning formulations was es- tablished and successfully utilized to confi rm DHA concentrations in the test articles. The sunless tanner market continues to grow rapidly, and our fi ndings can help to de- velop and evaluate new products with superior performance to fulfi ll consumers’ expecta- tions. ACKNOWLEDGMENTS We are grateful for the support of our colleagues at Ciba Corporation (part of BASF Group): Joseph Lupia, B. Scott Jaynes, Shawn O’Brian, and Sheila Loggins. REFERENCES (1) The Rose Sheet, 29(26), 9, (2008). (2) L. R. Robinson and P. R. Tanner, US patent 5,603,923, Artifi cial tanning compositions having improved color development (1997). (3) P. Lentini, N. Muizzuddin, E. Pelle, and L. Punto, US patent 5,503,824, Skin tanning compositions (1996). (4) P. D. Ziegler and B. A. Crotty, US patent 5,232,688, Self-tanner cosmetic compositions (1993). (5) O. Dueva-Koganov, T. Russo, and J. P. SaNogueira, US patent 7,378,084, Sunless tanning composition and method of sunless tanning (2008). (6) R. Jermann, M. Toumiat, and D. Imfeld, Development of an in vitro effi cacy test for self-tanning formu- lations, Int. J. Cosmet. Sci., 24, 1–8 (2002). (7) O. Dueva-Koganov, C. Rocafort, B. S. Jaynes, J. Lupia, B. Ridley, X. J. Zhou, and S. Barker, The impact of polymers in sunless tanning delivery systems (2007 SCC Annual Scientifi c Seminar), J. Cosmet. Sci., 59(2), 188–189 (2008). (8) http://www.ims-usa.com/ittrium/visit?path=A1x66x1y1xa0x1x65y1xc6x1x65y1xccx1x65 (9) N. Muizzuddin, K. D. Marenus, and D. H. Maes, Tonality of suntan vs. sunless tanning with dihy- droxyacetone, Skin Res. Technol., 6, 199–204 (2000). (10) P. A. Biondi, E. Passero, S. Soncin, C. Bernardi, and L. M. Chiesa, Selective determination of dihydroxy- acetone in self-tanning creams by HPLC as pentafl uorobenzyloxime derivative, Chromatographia, 65, 65–68 (2007). (11) A. Chardon, I. Cretois, and C. Hourseau, Colorimetric evaluation of the protection afforded by highly protective suntanning products, Proc. 16th IFSCC Cong., New York, poster presentation (1990).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)