JOURNAL OF COSMETIC SCIENCE 74 Dermatology Association, the Canadian Dermatology Association, and the American Medical Association. Mintel says in its recent report that users of sunless tanning prod- ucts in the U.S. are more receptive to new products compared with users of more mature personal-care categories and that a new entrant that produces signifi cantly better tanning results could make a signifi cant dent in the position of leading brands. Increasing aware- ness of the health risks associated with sun exposure motivated 39% of those surveyed to try sunless tanners. Among those consumers who have stopped using sunless tanners, 42% gave the reason that the products are too hard to apply, while 33% cited the prod- ucts’ “artifi cial” appearance (1). Different skin types may react differently with DHA due to the individual amino acid content, moisture level, skin tone, pH, and thickness. The result could be an uneven tan, one that is too dark or too light, or an orange color. It is known that various chemicals can modify or enhance the tanning reaction obtained with DHA on skin. Examples of such ingredients include amino acids (2), amino-substituted silicone compounds (3), polyacrylamide (4), amphoglycinate (amphoacetate) derivatives (5), thickeners, humectants, UV fi lters, vitamins, and emollients (6), and strong anti- oxidants (7). Certain thickeners, such as carbomer-type polyacrylates, when combined with DHA produced malodor and/or browning of the composition (4). However, the in- formation regarding the impact of rheology modifi ers on the development of sunless tan in vitro/in vivo and the analytical methods for DHA analysis in fi nished goods formula- tions is limited. Our objectives in this study were: • To evaluate and compare the ability of two DHA-containing sunless tanning formula- tions with similar excipients but different rheology modifi ers (blends), one with a cationic polymer-based rheology modifi er and the other with a polyacrylamide-based rheology modifi er, to infl uence the sunless color development in vitro and in vivo • To establish an analytical method to determine DHA concentration in sunless tan- ning formulations. MATERIALS AND METHODS TEST ARTICLES Rheology modifi ers were incorporated at 3% w/w levels (as supplied) in test formulations X and K, containing similar concentrations of DHA and the excipients: • Test article X with cationic polymer-based rheology modifi er [INCI: dimethylacryl- amide/ethyltrimonium chloride methacrylate copolymer (and) propylene glycol di- caprylate dicaprate (and) PPG-1 trideceth-6 (and) C10-11 isoparaffi n], recently introduced to the market by Ciba Corporation (part of BASF Group). • Test article K with polyacrylamide-based rheology modifi er [INCI: polyacrylamide (and) C13-14 isoparaffi n (and) laureth-7], from Seppic. The concentrations of active polymers are comparable in both commercial rheology mod- ifi ers (blends). The polyacrylamide-based rheology modifi er utilized in test article K was selected for this comparative evaluation because it was successfully used in sunless tanners demonstrating good effi cacy (4). Formulations of the test articles are presented in Table I.
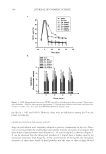
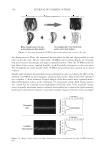
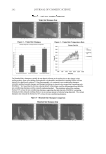
SUNLESS TANNING FORMULATIONS 75 IN VITRO/IN VIVO EFFICACY EVALUATIONS—GENERAL APPROACH An in vitro effi cacy testing methodology for evaluation of sunless tanners described by Jermann et al. (6) with our modifi cations (7) was utilized pre-hydrated Vitro Skin® (N-19) (8) was used as a substrate. This in vitro methodology is a reliable tool to predict the effi cacy and differences of self-tanning formulation performance on human skin (5–7). For the analysis of skin color in vivo after the application of test articles, the “natural uni- verse of suntan” and “natural universe of suntan tonality” realms were from Muizzuddin et al. (9), describing “…a cluster plane encompassing the distribution of normal skin tan- ning color representing the ‘natural universe’ of skin tanning or a response region within which natural skin tan color was observed.” PROCEDURE—IN VITRO The substrate was pre-cut into 4-cm by 4-cm pieces and pre-hydrated according to IMS-USA protocol (8). The application dose was 2 mg/cm2 temperature: 76°–78°F. Each piece of substrate was prepared for the experiment by uniformly applying 0.032 grams (2 mg/cm2) of test article on the surface with a pre-saturated fi nger cot. The substrate was then placed in a slide frame and put in the color development chamber. Three slides for each test article were used. The test articles were coded and their compositions were revealed after the in vitro study was completed. The colors of the samples were evaluated every 24 hours (three measurements per slide) for four days with a ColorTec-PSMTM Colorimeter: Observer 10° primary illuminant D65 CIE (1976) L*a*b* color space with tri-stimulus color values: L* (lightness), a* (red-green axis), and b* (yellow-blue axis). The difference in C* (chroma/saturation) is calculated according to the following equation: dC da db * ( * * ) = + 2 2 Table I Description of Test Formulations: X(with cationic polymer-based rheology modifi er) and K (with polyacrylamide-based rheology modifi er) %w/w Ingredient: INCI name X K Water 89.65 89.65 Pentylene glycol 4.00 4.00 Dihydroxyacetone 3.00 3.00 Phenoxyethanol (and) methylparaben (and) ethylparaben (and) butylparaben (and) propylparaben (and) isobutylparaben 0.35 0.35 Dimethylacrylamide/ethyltrimonium chloride methacrylate copolymer (and) propylene glycol dicaprylate/dicaprate (and) PPG-1 trideceth-6 (and) C10-11 isoparaffi n 3.00 — Polyacrylamide (and) C13-14 isoparaffi n (and) laureth-7 — 3.00 Sodium hydroxide, 10% aqueous solution q.s. q.s. pH 3.90 3.76
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)