JOURNAL OF COSMETIC SCIENCE 76 The total difference between two colors in CIE space is described as ΔE* ab and provides an integrated measurement of both chroma and lightness/darkness changes: dE ab dL da db * [( * ) * ) ( * )] = 2 2 2 PROCEDURE—IN VIVO In vivo studies were conducted on fi ve panelists using the volar aspects of their forearms as application sites. Application areas were 50 cm2. Before application of the test formula- tions, all panelists washed their forearms with Dove bar soap, rinsed with water, and pat- ted their forearms dry with paper towels. A period of 15 minutes was allowed before conducting the initial measurement of the skin color on marked application areas (fi ve measurements per area) using the ColorTec-PSM Colorimeter. The test articles were coded to hide their identity from those used in the in vitro studies, and their compositions were disclosed after the in vivo study was completed. Each test article was applied once on each panelist. The application dose was 2 mg/cm2, with an exact application amount of 0.1 g per application area. Left and right forearms were alternated to randomize the ap- plication sites for the test articles. The products were applied using fi nger cots pre-satu- rated with test article. Color measurements were taken at 24, 48, and 120 hours after a single application. Between measurements panelists were asked to follow their regular hygiene routine, taking showers, washing hands, etc. MATERIALS AND EQUIPMENT USED IN THE IN VITRO/IN VIVO STUDIES • Vitro-Skin® (N-19) (Lot# 8302), foam block and glassless slide mounts from IMS, Inc. • Powder-free class 100 fi nger cots (Lot# FOY8) from Fisher Scientifi c • ColorTec-PSM Colorimeter from ColorTec. HPLC METHOD An analytical method for the determination of the concentrations of DHA in sunless tanning formulations was developed based on the method described by Biondi et al., HPLC analysis of a pentafl uorobenzyloxime derivative (10) with our modifi cations. Sample preparation for HPLC. The samples were prepared for analytical testing by combin- ing 0.25 grams of each sample with 5 ml of sodium chloride (NaCl)-saturated aqueous solution, followed by vortex mixing for one minute. Then 5 ml of water was added to each sample and stirred for six hours. After that 25 μl of the sample and 50 μl of deriva- tizing agent O-(2, 3, 4, 5, 6-pentafl uorobenzyl) hydroxylamine hydrochloride 98% (Aldrich, 194484) were pipetted into a 10-ml volumetric fl ask and diluted using 50:50 acetonitrile and water. The standard used was DHA, cosmetic grade, from Napp Technologies LLC (Lot LL07- 2046). The preparation consisted in dissolving 0.0330 grams of the reagent in 10 ml of
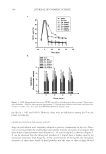
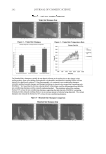
SUNLESS TANNING FORMULATIONS 77 citrate buffer. The citrate buffer was prepared by adding about 0.9 grams of the citric acid to 100 ml of water, titrated to pH 4.0 using a 0.1 N aqueous solution of sodium hydroxide (NaOH). Standard preparation for known in-house formulations. A base was provided and used to spike with a known amount of standard, used for the standard curve. The base was treated like a sample that was stirred and agitated with NaCl-saturated aqueous solution and water for six hours and then spiked with a known concentration of the standard. A standard linear curve was prepared as follows: 0.0895 grams of the standard was weighed in a 50-ml volu- metric fl ask, 10 ml of the saturated solution of NaCl was added, and the remaining volume was fi lled with water. Amounts of 2 μl, 5 μl, 10 μl, 15 μl, and 20 μl were pipetted into 20-ml scintillation vials. Fifty microliters of derivatizing reagent was added. Then 10 ml of an acetonitrile:water (50:50) solution was added to the scintillation vial. The derivati- zation reaction was completed after fi ve minutes at room temperature. HPLC parameters. An Agilent 1100 series HPLC with a quaternary pump and UV/Vis DAD detector with a detection wavelength at 262 nm was used. The column utilized was a Discovery 5-μm C18 column, 25 cm × 4 mm, from Supelco (Cat.# 504971-40), with an injection volume of 35 μl. The fl ow rate was set a 1 ml/min at 45°C. The run time was 25 min. An acetonitrile: water (50:50) solution was the mobile phase (isocratic). The re- tention time for the DHA-derivatized complex was 5.3 min. ANALYTICAL RESULTS AND DISCUSSION This HPLC method works well with the known sample matrix base. Our modifi cations of the method of Biondi et al. (10) included the following changes: a matrix base (placebo) and samples were pre-mixed with an NaCl saturated solution and water, and stirred for six hours. The base matrix was spiked with a known amount of standard (DHA) and used for quantitation. It is advantageous to prepare the standards in a known matrix since it eliminates the possibilities of interference from that matrix. This method also gives reli- able data for similar types of commercial formulations. A liquid–liquid extraction by dichloromethane of the commercial samples, for which the base matrix is not available, can be effective for free DHA extraction and the removal of base ingredients (10). The column temperature for the analyses was increased to 45°C for an improved peak shape of the DHA component. The HPLC chromatograms are presented in Figure 1, the results of DHA analysis in test articles are depicted in Table II, and a correlation between the DHA added to the base matrix and the DHA found is shown in Table III. IN VITRO EXPERIMENTAL DATA AND DISCUSSION In vitro experimental data are presented in Figures 2-4 below. As shown in Figure 2, both test articles showed similar trends in total color (ΔE*ab) changes vs time. Figure 3 shows the differences in the yellow (Δb*) and the red (Δa*) values after 72 hours of test article application. According to Jermann et al. (6), this particular time interval provides good correlation between relative tanning responses in vitro/in vivo. Noticeable variations in color depending on the test article were observed. Yellow/red balances varied, depending on the test article: X generated more red/same yellow color compared to K. As illustrated in Figure 4, ΔL* values after 72 hours were darker with the same ΔC* values X vs K. In
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)