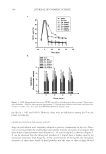
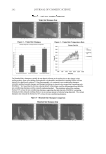
JOURNAL OF COSMETIC SCIENCE 112 preserved when bacteria are reduced by more than 99% (2 log) after two days and more than 99.9% (3 log) after seven days yeasts and molds should be reduced by more than 99% (2 log for criterion A and 1 log for criterion B) after 14 days. MICROBIOLOGICAL QUALITY IN TWO STATES OF USE: INTACT PRODUCT AND FOLLOWING THREE WEEKS OF USE The collected samples of cosmetic products were analyzed for total aerobic plate count (S. aureus, P. aeruginosa, E. coli, A. niger, and C. albicans) in two different states of use, the intact product and after three weeks of use. One gram or 1 ml of test sample was serially diluted in Letheen broth and plated in triptic soy agar and Sabouraud dextroze agar for bacteria and fungi, respectively. Plates were incubated at 35°C for bacteria and at 25°C for fungi. After a fi ve-day incubation, colonies of bacteria and fungi were counted and (cfu/g) calculated. The experiments were performed in triplicate. In some cases A. niger was identifi ed as black colonies with the characteristic morphology of actinomycetes. RESULTS MICROBIOLOGICAL QUALITY OF THE TEST PRODUCT The results regarding the microbiological safety of the formulations tested (challenge test according E. Ph., intact products, and following three weeks of use) are summarized in Tables IV, V, VIa–e, VII and VIII and in Figures 1a–e and 2. Table IV Challenge Test Criteria Regarding Aqueous and O/W Formulations Containing Preservative Systems I-VIII Type formulation Water content (%) Water activity (aw) pH Preservative system Challenge Test criteria (E. Ph.) Physicochemical stability Tonic lotion 95 — 5.5 I B regarding A. niger and C. albicans OK — 5.5 II A No — 5.5 III A No 90 0.9 5.5 IV A OK Shampoo 74 0.932 5.5 V A OK Shower gel 65 0.893 5.5 V A OK Conditioning cream 67 0.903 5.5 V A OK Anticellulite cream 65 — 5.5 V B regarding A. niger OK 0.903 5.5 VI A OK — 5.5 VII A No Cleansing milk 65 — 5.5 V B regarding A. niger OK — VI A No 0.919 VII A OK — VIII A OK Peeling cream 47 — 5.5 V B regarding A. niger OK 0.865 5.5 VI A OK — 5.5 VII A No —Not done.
ALTERNATIVES TO COSMETICS PRESERVATION 113 Staphylococcus aureus. Systems I–IV preserved effectively the high-water-containing tonic lotion against this strain (Table IV). System V could not be used in the case of the tonic lotion due to solubility problems, but it was active in the cases of shampoo and shower gel and all the emulsifi ed formulations tested (Figure 1a, Tables IV and VIa). Preservative systems VI and VII showed excellent activity in all the O/W formulations, i.e., the anti- cellulite cream, cleansing milk, and peeling cream. System VIII with the reduced per- centage (0.1% w/w) of levulinic acid also proved to be effective. In all above cases, criterion A of the E. Ph. were fulfi lled. Furthermore, no contamination from this strain was found, either in the intact products or following consumer use (Tables V, VII and VIII). Pseudomonas aeruginosa. This Gram-negative microorganism was susceptible to preserva- tive systems I–IV in the case of the tonic lotion (Table IV). System V effectively pro- tected the shampoo, shower gel, and O/W preparations. (Figure 1b, Tables IV and VIb). System VI also preserved equally to system V all the O/W formulations tested, i.e., the anticellulite cream, cleansing milk, and peeling cream. As with the previous microorgan- ism, levulinic acid (0.3% w/w, system VII, or 0.1% w/w, system VIII), was effective. In all of the above cases criterion A of the E. Ph. was met. Additionally, no contamination was found either in the intact products or following three weeks of use (Tables V, VII, and VIII). Escherichia coli. The population of this test microorganism seemed to be effectively con- trolled by systems I–IV in the case of the tonic lotion, since criterion A of E. Ph. was satisfi ed (Table IV). System V was also active in the shampoo and shower gel and the Table V In-Use Study (intact product and following three weeks of use) of Aqueous and O/W Formulations Containing Preservative Systems I-VIII Type of formulation Preservative system Type of container Total aerobic count (cfu/g) in intact product Total aerobic count (cfu/g) following three weeks of use Identifi ed microorganisms Tonic lotion I Glass bottle, pump 10 10,000 A. niger II Glass bottle, pump 10 10 Absence III Glass bottle, pump 10 10 Absence IV Glass bottle, pump 10 10 Absence Shampoo V PE soft-touch bottle, pump 10 10 Absence Shower gel V PE soft-touch bottle, pump 10 10 Absence Conditioning cream V PE soft-touch bottle, pump 10 10 Absence Anticellulite cream V Glass bottle, pump 10 9000 A niger VI Glass bottle, pump 10 10 Absence VII Glass bottle, pump 10 10 Absence Cleansing milk V Glass bottle, pump 10 8800 A. niger VI Glass bottle, pump 10 10 Absence VII Glass bottle, pump 10 10 Absence VIII Glass bottle, pump 10 10 Absence Peeling cream V Glass jar, PP cap 10 9500 A. niger VI Glass jar, PP cap 10 10 Absence VII Glass jar, PP cap 10 10 Absence PE = polyethylene PP = polypropylene.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)