JOURNAL OF COSMETIC SCIENCE 126 synthesis, and the increased expression of their degradative enzymes, especially collage- nases (MMP-1), gelatinases (MMP-2, MMP-9), and elastases (1–14). The cosmetic industry is active in identifying natural products to counteract skin aging. Many of these products or actives have antioxidant and/or anti-infl ammatory properties, and are rich in polyphenols or fl avonoids (15–26). A fl avonoid that has been extensively investigated for its antimicrobial and anticarcinogenic properties, but not for its anti- skin-aging potential, is xanthohumol from the hop plant Humulus lupulus L. (Cannabi- naceae) (hops) (27–31). It has potent anti-infl ammatory and antioxidant properties, including inhibition of the NF-kB transcription factor and nitric oxide production by infl ammatory cytokines (27–31). Xanthohumol’s potential for anti-skin-aging has not been reported. We evaluated the effi cacy of xanthohumol in (a) inhibiting elasatase, MMP-1, MMP-2, and MMP-9 activ- ities (b) stimulating expression of types I, III, and V collagens, elastin, fi brillin-1, and fi brillin-2. MATERIALS AND METHODS MATERIALS The materials used were the following: enzymes [elastase (Elastin Products Co. SE563), MMP-1 (Biomol Se-180), MMP-9 (Biomol Se-244), MMP-2 (Biomol Se-109)] substrates [elastase (Bachem I-1270), MMP-1 (Bachem, M-2055), MMP-2,9 (Bachem M-1855)] dermal fi broblasts (Cascade Biologics) escort (Sigma) protein detector kit (KPL Co) antibodies/standards [collagen (Chemicon), elastin (Elastin Products Co)] plasmids (gifts from Dr. Joel Rosenbloom, School of Dental Medicine, University of Pennsylvania, PA) dual luciferase reporter assay (Promega) xanthohumol (gift from Dr. Gearhaard Haas, Fairleigh Dickinson University, NJ) and positive controls [ascorbic acid (AA, Sigma), EDTA (Sigma), PMSF (Sigma), protease inhibitor (Roche)] ELASTASE OR MMP ACTIVITY CALIBRATION AND INHIBITION The enzymes (elastase, MMP-1, MMP-9, and MMP-2) were calibrated by reacting two- fold serial dilutions of each of the enzymes (starting concentration of 1 μg/μl) with respec- tive substrates (0.5 mM) in incubation buffer (elastase: 0.09 M Tris-0.5 M NaCl buffer MMPs: 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM CaCl2). The reaction kinetics was measured fl uorometrically (355 excitation/450 emission) every fi ve minutes for a total length of 1.5 hours. The optimal enzyme concentrations (linear dose response) were determined to be 0.1 μg/ml for elastase, 0.77 μg/μl for MMP-1, 0.5 μg/μl for MMP-2, and 0.1 μg/μl for MMP-9. Xanthohumol (0.001%, 0.01%, 0.1%, and 1% of a stock of 50 mg/ml) (Xan) or positive controls (10 mM PMSF, 5m MEDTA, 0.5 mM ascorbic acid or 1XProtease inhibitor) were incubated with the optimal concentration of each of the enzymes in incubation buffer for ten minutes followed by the addition of the respective substrate. The activities of elastase and each of the MMPs were measured fl uorometrically at 355 excitation/450 emission.
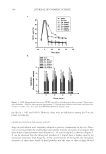
REGULATION OF ECM REMODELING BY XANTHOHUMOL 127 EXPRESSION OF FIBRILLAR COLLAGENS, ELASTIN, AND FIBRILLINS Dermal fi broblasts were cultured in complete Dulbecco’s Modifi ed Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin/ streptomycin, and 1% L-glutamine (Sigma). For the experiments, cells were trypsinized, seeded in 33-mm dishes, transfected with COL1A1 promoter (or elastin promoter), fi refl y luciferase (pGL4 vector), and TK-hRenilla luciferase plasmids with escort, and dosed with or without (control) xanthohumol (0.01%, 0.1%, 1%) or 2.5 mM ascor- bic acid for 24 hours in basal media containing 1% serum replacement (Sigma) (19–21). The cells were examined for viability by MTS assay (Promega). The media were exam- ined for collagen (types I, III, and V) and elastin fi ber components (tropoelastin, fi brillin-1, and fi brillin-2) with respective antibodies by indirect ELISA (19–21). For ELISA, ali- quots of media (from experiments) or respective standards were added to 96-well plates and incubated overnight at 4°C. The wells were blocked with bovine serum albumin and then incubated with the respective antibodies for one hour at room temperature. The plates were washed thoroughly with wash buffer, incubated with secondary antibody linked to peroxidase for one hour at room temperature, washed with wash buffer thoroughly, and subsequently incubated with peroxidase substrate until the development of color, which was measured spectrophotometrically at 405 nm. The data were normalized for cell via- bility. The cells were lysed and measured for luminescence from fi refl y and renilla luciferase activities with specifi c substrates (Promega). The luminescence from fi refl y luciferase was normalized with that from renilla luciferase. DATA ANALYSIS The effects of xanthohumol or positive controls are represented as a percent of control (no additives) represented at 100%. The data were statistically analyzed for signifi cant differ- ences by ANOVA and Student t-tests at the 95% confi dence interval. Signifi cant effects of xanthohumol, relative to control, are represented by * in the fi gures. RESULTS AND DISCUSSION INHIBITION OF ELASTASE AND MMP-1, 2, AND 9 ACTIVITIES BY XANTHOHUMOL The ECM damaging enzymes are produced by epidermal keratinocytes, dermal fi bro- blasts, and neutrophils, which are the predominant source for photoaged skin (9–14). While collagen is degraded by MMP-1 and MMP-2, the elastic fi bers are degraded by elastase, MMP-9, and MMP-2 (7–9). Xanthohumol inhibited the activities of elastase and MMP-9 at all concentrations (0.001–1%), and MMP-1 and MMP-2 at the higher concentrations (0.1% and 1%), suggesting a greater benefi t to the elastin fi bers (Figure 1a–d). There was complete inhibition of each of the enzyme activities by 1% xanthohu- mol. The concentrations of xanthohumol that inhibited elastase, MMP-9, MMP-1, and MMP-2 activities by 50% were extrapolated as 0.001%, 0.05%, 0.25%, and 0.5%, re- spectively.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)