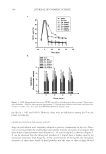
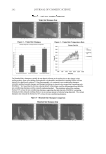
J. Cosmet. Sci., 61, 125–132 (March/April 2010) 125 Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fi brillar collagens, elastin, and fi brillins by xanthohumol NEENA PHILIPS, MATHEW SAMUEL, ROSEMARIE ARENA, YU-JUN CHEN, JENNIFER CONTE, PRASHANTI NATRAJAN, GERHARD HAAS, and SALVADOR GONZALEZ, School of Natural Sciences, Fairleigh Dickinson University, Teaneck, NJ (N.P., M.S., R.A., Y.-J.C., J.C., P.N., G.H.), Industrial Cantabria Farmaceutica, S.A, Madrid, Spain (S.G.), and Dermatology Service, Memorial Sloan- Kettering Cancer Center, New York (S.G.). Accepted for publication August 24, 2009. Synopsis In skin aging there is deterioration of the extracellular matrix’s collagen and elastin fi bers, from its reduced biosynthesis and increased degradation by elastase and matrixmetalloproteinases (MMPs). Xanthohumol is a fl avonoid isolated from the hop plant Humulus lupulus L., with anti-microbial, antioxidant, anti-infl ammatory, and anti-carcinogenic properties. The goal of this research was to investigate xanthohumol as an anti-skin- aging agent via its benefi cial regulation of the extracellular matrix. To this purpose, we examined the direct effect of xanthohumol on the activities of elastase and MMPs (MMPs 1, 2, and 9) and its effect on the expres- sion (protein and/or transcription levels) of collagens (types I, III, and V), elastin, and fi brillins (1 and 2) in dermal fi broblasts. Xanthohumol signifi cantly inhibited elastase and MMP-9 activities from its lowest con- centration, and MMP-1 and MMP-2 at its higher concentrations, which implies a greater protective effect on elastin. It dramatically increased the expression of types I, III, and V collagens, and elastin, fi brillin-1, and fi brillin-2 in dermal fi broblasts. The effects were similar to those of ascorbic acid. This is the fi rst report identifying xanthohumol’s potential to improve skin structure and fi rmness: it simultaneously inhibits the activities of elastase/MMPs and stimulates the biosynthesis of fi brillar collagens, elastin, and fi brillins. INTRODUCTION Alterations in collagen and elastin, which form the extracellular matrix (ECM), are re- sponsible for the clinical manifestations of skin aging, which are wrinkles, sagging, and laxity (1–6). The fi brillar collagens, types I, III, and V, in the order of predominance, provide structure whereas elastin forms elastic fi bers with fi brillin to gives skin fi rmness and elasticity. The atrophy of collagen and elastin fi bers in skin aging is from reduced Address all correspondence to Neena Philips.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)