JOURNAL OF COSMETIC SCIENCE 148 cuticle cells has been suggested to be covered by a layer of covalently bound fatty acids, a major component of which is 18-methyleicosanoic acid (18-MEA). 18-MEA is an un- usual branched-chain fatty acid, covalently bound, via thioester or ester linkages, to the cuticle surface of hair fi bers (2–5). The absence of 18-MEA is considered one of the rea- sons for an increase in interfi ber friction, and it may have an infl uence on sensory percep- tions of hair, such as a dry feel and diffi cult combing (6). For decades, many studies have been made to investigate the roles of 18-MEA on the surface properties of hair. FFM is a powerful technique for the investigation of surface properties, including wetting and tribological properties, and has revealed that 18-MEA makes the surface hydrophobic and acts as a boundary lubricant to decrease frictional re- sistance (6–10). Dynamic contact angle measurement is another useful method to exam- ine the changes in surface hydrophobicity and has indicated that when 18-MEA is removed, the surface of hair becomes hydrophilic (11–14). In this study, we have investi- gated the effects of the removal of 18-MEA on the surface properties in wet and dry en- vironments and the role of 18-MEA on hair alignment and appearance. EXPERIMENTAL MATERIALS Hair samples. Hair fi bers, kindly provided by 48 Japanese females, were cut at the root end, just above the scalp. Twenty-three subjects had chemically untreated hair, and the hair of the others had been treated by bleaching, coloring, and/or permanent waving. The hair fi bers for our specifi c experiment were kindly provided by a Japanese female aged 40. The fi bers were cut at a distance of approximately 20 cm from the root end on the back of her head. The hair had never been treated with any chemical agents, such as bleaches, hair coloring, or permanent-waving solutions. Preparation of 18-MEA-removed hair. The hair fi bers were treated with a solution of 0.1 M potassium t-butoxide in t-butanol for fi ve minutes at room temperature and at a liquor:fi ber ratio of 10:1. The alkali was then removed by rinsing the hair with t-butanol (2×), ethanol, and fi nally, by washing in water. METHODS Semi-quantitative analysis of 18-MEA. Semi-quantitative analysis of 18-MEA adsorbed on the outermost surface of the hair fi ber was measured by a TOF-SIMS IV instrument (ION-TOF GmbH, Germany) using 25-keV Bi32+primary ions (average current 0.13 pA, pulse width 23.0 ns, repetition rate 10 kHz) in high-current bunched mode. The analysis area of 50 × 50 μm was randomly rastered by primary ions and was charge-compensated by low-energy electron fl ooding. The amount of 18-MEA was expressed as the relative ion yield of 18-MEA versus the CN ion yield, which was derived from hair proteins. It is known that the ion yield of 18-MEA detected by TOF-SIMS changes under the infl uence of not only the amount of 18-MEA on the surface of the hair but also sample conditions, surface charging, and instrument condition. For the comparison of the ion yield of 18-MEA among samples, the peak was generally normalized by the standard ion peak,
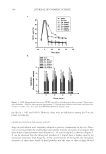
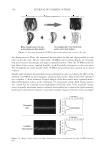
18-MEA AND HAIR APPEARANCE 149 such as the sum of the total ion yield, the ion peak from hydrocarbons, or the ion peak from the matrix constituent. In the case of using the sum of the total ion yield or the ion peak from hydrocarbons as a standard ion, however, these yields change due to surface contaminants such as anionic surfactants and silicone oil. Here, the ion peak from the matrix constituent was chosen as a standard ion to avoid these effects. In this study, the CN ion was used for normalization since the matrix of the hair surface is keratinous protein and the CN ion was strongly detected in the TOF-SIMS measurement of hair samples. Measurement of surface properties of hair. The wetting forces of hair were measured by the Wilhelmy method using a K100MK2 tensiometer (Kruss). Single hair fi bers were scanned over 3 mm at a velocity of 2 mm/min for the advancing mode. Dynamic contact angles were calculated from F cosθ = Sdγ where F is the wetting force, d is the diameter of the hair, γ is the surface tension of water, and θ is the contact angle of the liquid versus the fi ber surface. The hair fi ber diameter was measured on the transverse section of each fi ber with a rotating fi ber diameter measurement system equipped with a laser (Kato Tech Co.) at 20°C and at a relative humidity (RH) of 65%. The wetting force measurements were also performed at 20°C, 65% RH. Frictional properties of the outermost surface of the hair in the wet environment were measured using a Nanoscope III Dimension 3000 (Veeco Instruments, Santa Barbra, CA). Friction force microscopy (FFM) data were acquired using unmodifi ed silicon nitride (Si-N) cantilevers (spring constant of 0.12 Nm-1). After engagement of the tip with the cuticle surface, the tip was scanned parallel to the longitudinal axis of the fi ber. In order to minimize scanning artifacts, a scan rate of 1 Hz was used for all measurements. To characterize frictional properties, 2- × 2-μm scans of the cuticle faces (without edges) were performed. Trace and retrace FFM datasets were obtained, and the friction force was calculated by subtracting the retrace FFM signal from the trace FFM signal. In the FFM measurement of the cuticle surfaces before and after removing 18-MEA, trace and retrace FFM datasets were obtained at different areas of the cuticle surfaces samples were ran- domly picked up from untreated hair and 18-MEA-removed hair, and trace and retrace FFM datasets were obtained. RESULTS AND DISCUSSION INFLUENCE OF SCALE DIRECTION OF HAIR ON DYNAMIC CONTACT ANGLE A sensitive method to provide information on the outermost surface of hair is contact angle measurement. Its interpretation, however, is not always straightforward because surfaces usually give two stable contact angles, advancing and receding. The difference between the advancing and receding contact angles is referred to as contact angle hyster- esis. It is generally accepted that the contact angle hysteresis can be due to a multitude of effects, such as surface roughness, chemical heterogeneity, surface deformation, and sur- face confi guration changes (15–19).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)