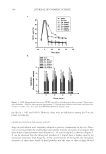
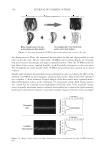
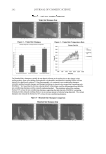
JOURNAL OF COSMETIC SCIENCE 158 the drying process. Then, the untreated dry hair fi bers lie fl at and align parallel to each other in the dry state. On the other hand, 18-MEA-removed hairs (Figure 14) entangle and are not easy to disentangle and align in a parallel manner. Then, the 18-MEA- removed hair fi bers form coarser, tangled bundles, with disorderly alignment in the wet state. The entangled part of the bundle of the 18-MEA-removed hair dries quickly and is fi xed in place. Finally, light intensity measurements were performed in order to evaluate the effect of the removal of 18-MEA on the luminance along the hair tresses. Hair tresses were mounted on a cylinder, 4 cm in diameter. Digital images of the hair tresses were captured with a Nikon D50 digital camera, with a resolution of 3 megapixels, using a fl ash. Image analysis was carried out by scanning across highlighted and dark areas of the resultant image, using originally developed analysis software that enabled us to obtain the light intensity (luminance) distribution. Figure 15 provides example images of the hair tresses and light Figure 15. Images of hair tresses and light distribution curves for untreated hair and 18-MEA-removed hair. Figure 14. Schematic diagram of 18-MEA-removed hair going from a wet to a dry state.
18-MEA AND HAIR APPEARANCE 159 distribution curves for untreated hair and 18-MEA-removed hair. It was apparent that the removal of 18-MEA decreases the contrast between specular refl ection and other re- gions, which is due to the disorderly alignment of the hair fi bers. Broadening of specular refl ection, a luminance intensity of 88 and 18 pixels of half bandwidth, and approxi- mately a 20% decrease in luminance intensity were evident in the case of 18-MEA- removed hair, while the specular refl ection of the untreated hair was relatively sharp, having a luminance intensity of 115 and ten pixels of half bandwidth. The results revealed that the removal of 18-MEA decreases hair gloss. CONCLUSIONS The decrease in 18-MEA on the cuticle surface affects the hydrophobic–hydrophilic prop- erties of hair by providing lower advancing and receding contact angles. An important cosmetic role of 18-MEA is to allow hair fi bers to lie fl at and parallel with respect to each other in wet environments by providing relatively high receding contact angles and low surface friction. Hair alignment in the dry environment, which infl uences hair luster, is directly affected by hair alignment in the wet environment, particularly in the case of damaged hair. REFERENCES (1) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 4th ed. (Springer-Verlag, New York, 2002). (2) A. P. Negri, H. J. Cornell, and D. E. Rivett, The nature of covalently bound fatty acids in wool fi bers, Aust. J. Agric. Res., 42, 1285–1292 (1991). (3) A. P. Negri, H. J. Cornell, and D. E. Rivett, Effects of proceeding on the bound and free fatty acid levels in wool, Text. Res. J., 62, 381–387 (1992). (4) S. Naito, M. Ooshika, N. Yorimoto, and Y. Kuroda, The structure of bound lipids of human hair fi bers and its physical properties, Proc. 9th Int. Wool Text. Res. Conf., Biella, Italy, II, 367–374 (1996). (5) D. J. Evans and M. Lanczki, Cleaavage of integral surface lipids of wool by aminolysis, Textile Res. J., 67, 435–444 (1997). (6) S. Breakspear, J. R. Smith, and G. Luengo, Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface, J. Struct. Biol., 149, 235–242 (2005). (7) V. Dupres, T. Camesano, D. Langevin, A. Checco, and P. Guenoun, Atomic force microscopy imaging of hair: Correlations between surface potential and wetting at the nanometer scale, J. Colloid Interface Sci., 269, 329–335 (2004). (8) V. Dupres, D. Langevin, P. Guenoun, A. Checco, G. Luengo, and F. Leroy, Wetting and electrical prop- erties of the human hair surface: Delipidation observed at the nanoscale, J. Colloid Interface Sci., 306, 34–40 (2007). (9) U. Kalkbrenner, H. Koener, H. Hoecker, and D. E. Rivett, Studies on the composition of the wool cu- ticle, Proc. 8th Int. Wool Text. Res. Conf., Christchurch, New Zealand, I, 398–407 (1990). (10) M. Huson, D. Evans, J. Church, S. Hutchinson, J. Maxwell, and G. Corino, New insights into the na- ture of the wool fi bre surface, J. Struct. Biol., 163, 127–136 (2008). (11) Y. K. Kamath, C. J. Dansizer, and H.-D. Weigmann, Wettability of keratin fi ber surfaces, J. Soc. Cosmet. Chem., 28, 273–284 (1977). (12) T. Baba, N. Nagasawa, H. Ito, O. Yaida, and T. Miyamoto, Changes in the covalently bound surface lipid layer of damaged wool fi bers and their effects on surface properties, Textile Res. J., 71, 308–312 (2001). (13) R. Molina, F. Comelles, M. R. Julia, and P. Erra, Chemical modifi cations on human hair studies by means of contact angle determination, J. Colloid Interface Sci., 237, 41–46 (2001). (14) R. A. Lodge and B. Bhushan, Wetting properties of human hair by means of dynamic contact angle measurement, J. Appl. Polym. Sci., 102, 5255–5265 (2006).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)