358 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS that the perspective for pressure packaging of all types will be furth. er ex- panded. BIBLIOGRAPHY (1) Croxton, Fred E., "Bibliography on the Solubility of Argon, Carbon Dioxide, Helium and Nitrogen in Organic Liquids," United States Atomic Energy Commission, AECU- 100 (K-339), Jan. 25 (1949). (2) "CSMA Aerosol Gui:le," Aerosol Div., Chem. Specialties Mfrs. Assoc., June (1957), 95 pp. (3) Drug Topics, May 12 (1958), p. 69. (4) Drug Trade News, April 7 (1958), pp. 30, 36, 43. (5) "Facts on Gases Used for Food Products," Air Reduction Co., Inc. (6) Lange, N. A., "Handbook of Chemistry," 8th Ed., Sandusky, Ohio, Handbook Pub- lishers, Inc. (1952). (7) Stetz, R. J., et al., U.S. Patent No. 2,815,889. (8) Urlaub, George, U.S. Patent No. 2,778,543. (9) Pyenson, H., U.S. Patent No. 2,723,200. (10) Shepherd, H. R., Food Processing, 19, 30 (1958). (11) Prussin, Samuel, et aL, U.S. Patent Applied for, Dec. 6, 1957. (12) Webster, Robert C., Modern Packaging, 31, 151-156, 206, 208 (1957). (13) "Solubilities of Gases in Edible Fats and Oils, Water and Sugar Solutions," Air Reduc- tion Co., Inc., Rept. No. AR-265. (14) Seidell, A., "Solubilities of Inorganic and Metal Organic Compounds," Princeton, D. Van Nostrand Co. (1940). (15) Ausband, John R., Eye, Ear, Nose Throat Monthly 36, 463 (1957). (16) Strobach, William, "Uses of Nitrous Oxide as Refrigerant Gas," presented at the 31st Annual Meeting Compressed Gas Mfrs. Assoc., Inc., Jan. 25, 1944. ULTRAVIOLET LIGHT ABSORBERS IN COSMETICS By A. SIGNORE and F. E. WooDw^RI)* Presented )tune 4, 1958, New York City ULTRAVIOLET nIOH'r, either alone or in conjunction with oxygen, is an important factor in the degradation of almost all organic materials. Putting it another way, most organic materials degrade sooner or later in the presence of ultraviolet light• In the past few years a new class of chemicals has appeared on the market. These chemicals are strong ultraviolet light absorbers but are themselves completely stable. They are designed to be used for the protection of commercial products such as plastics, wood, paint, cosmetics, fibers, etc., from the deleterious effects of ultraviolet light. Their use is analogous to that of the antioxi- dants. It is believed that derivatives of 2-hydroxy benzophenone are the most stable and the most efficient compounds for this use and are now marketed under the tradename "Uvinuls, "© "Cyasorb "© and "Light * General Aniline & Film Corp., Central Research Laboratory, Easton, Pa.
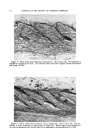
ULTRAVIOLET LIGHT ABSORBERS IN COSMETICS 359 Absorber"©.t Ultraviolet light absorbers with different basic structures have very recently appeared on the market and appear in most cases to be as stable and as efficient as the benzophenones but they have not been studied as thoroughly or had a chance to become widely used. Ultraviolet light absorbers are, of course, universally used in suntan lotions. These products are designed to absorb radiation below about 310-320 millimicrons. Their stability to light is a desirable but not an all-important feature of these products, and suntan preparations will not be discussed in this paper. To act as more or less permanent pro- tective agents for organic materials, it is necessary that the ultraviolet light absorber itself be stable for at least as long as it is supposed to protect. Also, the ultraviolet light absorbers under discussion absorb very strongly in the region of 280-320 millimicrons and would, if used on the skin, completely prevent suntanning. Ultraviolet light is normally defined as the radiation below 400 milli- microns, which is the lower limit of light visible to the human eye (Fig. 1). Ultraviolet radiation from the sun reaching the earth ranges from 280 millimicrons on up. The earth's atmosphere filters out a good deal of t "Uvinul" is a registered trademark of General Aniline and Film Corporation. "Cyasorb" is a registered trademark of American Cyanamid Company. "Light Absorber" is a registered trademark of The Dow Chemical Company. STABLE ULTRAVIOLET ABSORBERS OF 2 HYDROXYBENZOPHENONE TYPE HO 0 2,4 dlhydroxy -- HO n OH -•- 2,2.', 4,4' tetrahydroxy -- 2 htdroxyt•,5 dlchloro -- 2 hydroxy, 4 methow -- c,,o•../ •..../oc,, 2.•dlhydroxy 4,4'dlmetho•y- 2 hydroJr,/, 4111Mho•, 5 lulfo -- Figure 1.--Stable ultraviolet absorbers of 2-hydroxybenzophenone type.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)