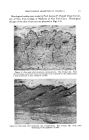
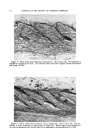
362 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS degradation is almost always catalyzed by ultraviolet light. Other systems which catalyze free radical reactions, such as the ferrous-ferric and cuprous-cupric redox systems, also catalyze free radical oxidation and frequently obscure the results of practical laboratory tests (4). In general, it is suggested that the combined use of the appropriate ultraviolet light absorber and antioxidant should show a synergistic effect. The ultraviolet light absorber will retard the formation of reactive radicals and the antioxidant will prevent the further reaction with oxygen of those that do form. From the other side of the picture, let us consider the mode of action of the light stable ultraviolet absorbers. Figure 1 gives the structures of six such products. Each of these products has the 2-hydroxy benzo- phenone basic structure, and each has been found to be exceptionally stable to the action of ultraviolet light. Figure 2 shows the ultraviolet spectra of three benzophenones (5). The strong absorption of 2,4-dihydroxy benzophenone in the region of 370 microns is explained by the resonating hydrogen bridge six-membered ring structure, which can be formed with 2-hydroxy benzophenone and its isomers. Not only is the absorption of the 2-hydroxy benzophenone structures fundamentally different, but their chemical reactivity is as well. The 2-hydroxy group.is much less readily acetylated or etherified STABILITY TO ULTRAVIOLET CH._O • OCH] 10% IN 5MIL FILM CELLULO6E ACETATE A-- UNEXPOSED B-- 60 HOURS-FADE:OMETER ß I I I I I I I 220 260 300 :540 $80 420 WAVELENGTH IN MILLIMICRONS lOO8090 at_ 70 so _•' 50 - $0 - 20 ø I0 I I 46O Figure 4.--Stability to ultraviolet light of 2•2',4,4'-tetramethoxybenzophenone.
ULTRAVIOLET LIGHT ABSORBERS IN COSMETICS 363 than is the 4-hydroxy group. This resonating six-membered ring is apparently the stabilizing electron link responsible for the peculiar light stability of these structures. The stability of these absorbers to ultraviolet light is illustrated by Fig. 3 which shows the transmission curve, 2,2'-dihydroxy-4,4'-dimethoxy benzophenone to be unchanged after 1000 hours' exposure to ultraviolet light. The importance of the 2-hydroxy benzophenone structure to the stability of these products is illustrated by Figs. 3 and 4 which show the almost complete decomposition of the corresponding tetramethoxybenzophenone in 60 hours in an Atlas Fadeometer. There are numerous other examples of this phenomenon. The structure in Fig. 3 and the corresponding 2,2', 4,4'-tetrahydroxybenzophenone are the most stable of all UV light ab- sorbers discovered to date. In general, compounds of the structure: R 2 OH where R' = hydrogen or C• to C•8 alkyl R2 = and R4 = H, C1, OR' or a C•-C•s alkyl exhibit the desirable properties discussed in this paper. These compounds might be useful in a variety of cosmetic preparations such as face powder, rouges, face creams, foundation creams, lipsticks, fingernail lacquers or coatings, dyed or colored toilet waters, perfumes, colognes, brilliantines and bath salts. In addition to inherent light stability, the most important feature for a useful UV absorber is high absorption power in the ultraviolet region and no absorption power in the visible region (i.e. the product should have no color). The absorption curve for 2,4-dihydroxy benzophenone peaks in the region of 300 and is negligible above 400. This means, in effect, that the material is colorless. A corresponding curve for 2,2',4,4'-tetrahydroxy- benzophenone would have a slight amount of absorption above 400 mil- limicrons. This means that the products may have a slight yellow cast, depending on the relative concentration of UV absorber present. Solubility is perhaps the next most important criterion from a practical standpoint (see Fig. 5). In general, it may be stated that 2 4-dihydroxy benzophenone and 2,2',4,4'-tetrahydroxy benzophe- none are the most alcohol-soluble members of this group, and 2,2'-di- hydroxy-4,4"-dimethoxy benzophe- none has the best solubility in hydro- carbons. This is not to imply that the solubility of these materials is SOLUBILITY OF UVINUL D-50 Methyl Ethyl Ketone 22•o 50/50 Isopropanol/Water 11% Methyl Alcohol 50 •o Ethyl Alcohol 40(•o Butyl Alcohol 13 % Ethyl Acetate 20% Toluene 0.05 % Water {Room Temp.) 0.1% Water {59øC) 0.5% Figure 5.--Solubility of 2,2',4,4'-tetra- hydroxybenzophenone (Uvinul D-S0 is a registered trademark of General Aniline and Film Corporation).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)