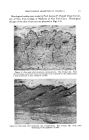
INFRARED SPECTROSCOPY IN COMPOSITION OF OILS 381 It often happens that one need go no further to identify a component. If the distillation fraction is sufficiently pure, the infrared spectrum of the fraction may be used directly to identify the component. If one can pick out several characteristic absorption peaks, one can go to the infrared reference file to try to match the spectrum to a reference. We consider that the matching of at least five peaks in the "fingerprint" region of the spectrum gives "tentative" proof of the presence of a particular compound. Confirmatory evidence can usually be obtained either by spectroscopy on a more highly purified sample or by means of other techniques. Match- ing of spectra means not only that the peaks must have the proper wave- lengths but also the correct shapes and relative heights. Spectrum 3A is that of alpha-pinene of about 90+ per cent purity, and Spectrum 3B is that of pure alpha-pinene. The points of similarity are quite clear. I I(b) Reference o•-Pinene I I I 13 14 I I I I I I I 4 5 6 8 9 10 11 12 Vfovelength, microns Figure 3.--Infrared spectra of isolate and of known •-pinene. On the basis of their infrared spectra, selected fractions from the distil- lation were pooled. In most of these pooled samples, there was a con- siderable infrared absorption in the carbonyl region. This led to the use of the Girard reagent for the separation of aldehydes and ketones. Figure
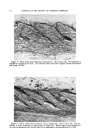
382 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (a) Starting }/alerial (b) Non-Carbonyl Compounds (c) Corbonyl Compounds I I [ I I I I I I I I I 3 4 5 6 7 8 9 10 II 12 13 14 15 Wavelength, microns Figure 4.--Infrared spectra of starting material and products from Girard separation. 4 illustrates the Girard separation. Spectrum 4A shows the pooled frac- tions with carbonyl band at 5.78 microns the next spectrum shows the same mixture with the aldehydes and ketones removed by the Girard reagent. In the latter, most of the carbonyl absorption has disappeared. The small amount remaining is caused by the presence of esters which are unaffected by the Girard reagent. However, the hydroxyl absorption at 2.98 microns also was decreased by the treatment. Correspondingly, the spectrum of the regenerated carbonyl compounds (4C) showed the presence of an alcohol which was identified as hexanol. It turned out
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)