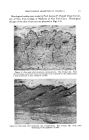
INFRARED SPECTROSCOPY IN COMPOSITION OF OILS 383 that the hexanol was carried along with the Girard complex because of its water solubility and appeared along with the regenerated carbonyl compounds as an artifact. By means of infrared spectra we were able to quickly detect the alcohol contaminating the carbonyl compounds. In subsequent experiments this difficulty was eliminated by a modification in the Girard procedure. This illustrates another virtue of following the separations by means of infrared spectra: many of the experimental difficulties which arise in carrying out these chemical and physical separations can be easily detected and corrected. Infrared spectra can also be of invaluable help in developing new sepa- ration methods. Because of the ability to make quantitative estimates as well as qualitative identifications from infrared spectra, one can easily determine the degree of separation of the components of a given mixture. It can then be used as a rapid and convenient criterion for comparing the efficiency of a series of separation processes. For example, we carried out a number of experiments to determine the best solvent, absorbent and other conditions for the chromatography of our samples, and infrared spectra were used to find just which set of conditions worked best. This could have been done by other means but not nearly so rapidly and reliably. This chromatographic separation was generally the next step in the procedure, and here again spectra were used extensively.• The starting materials for the chromatographic procedure were either the regenerated carbonyl compounds or the residual hydrocarbons, alcohols, esters, etc. The chromatographic technique is one which was developed as being particularly suitable for these mixtures and has proved to be very valuable in separating such groups as aliphatic hydrocarbons, terpenes, aromatic hydrocarbons, etc. Using this technique one can take a small sample and separate it into a large number of fractions. In order to avoid having to determine an inordinate number of spectra, a little discretion is necessary. By using odor as a guide, it is possible to pick out each separated compound. What one does is to collect each fraction, distill the solvent and note the odor and approximate volume of the residue. Using just a few properly chosen spectra, one can detect all of the components that are eluted. In chroma- tography, there is nothing akin to the possibilities for azeotroping that occur in distillation and the elution pattern is thus easily followed. Be- cause of their ability to characterize mixtures, just a few infrared spectra can reveal the whole pattern of the elution. In contrast to liquid chromatography, separations of an entirely dif- ferent kind are obtained with gas-partition chromatography or, more commonly, vapor-phase chromatography. Whereas liquid chroma- tography separates compounds on the basis of the strength with which
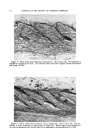
384 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS they are adsorbed by the solid column packing, gas-partition chroma- tography separates compounds primarily on the basis of their volatility and secondarily on the basis of their relative solubilities in the immobile liquid of the column packing. Since gas chromatography most con- veniently handles small quantities, it is commonly used on the fractions from the liquid chromatography. The above separation techniques are the major ones that have been used. Among the others used are clathrate (urea) complex formation and acid-base extraction. There are several other techniques which are of interest to workers in this field but which were not suitable to the partic• ular materials with which we were working. These include column partition chromatography, paper chromatography, liquid-liquid distri- bution and the use of "chromatostrips." I I 5 d • fo (a) Pooled Distillation Fractions1 11 I I I I I• 1•1 14 ' (b) Corbonyl CompoundI I I I I I I I I i 6 7 8 9 10 11 12 13 14 15 Wavelength, microns Figure 5.--Infrared spectra of pooled distillation fractions and carbonyl compounds. The identification of impure materials by infrared spectroscopy is not a clear-cut matter. One cannot say that a compound which is 85 per cent pure can be identified and one which is 80 per cent pure cannot. It depends entirely on the nature of the spectra of the compound and of the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)