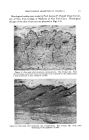
360 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ultraviolet light so that very little, if any, radiation below this level ever reaches the earth. This, of course, varies with altitude, with latitude, and with the seasons of the year. That ultraviolet is a problem is evidenced all around us fibers get brittle, colors fade, perfumes darken, some woods darken, others bleach, plastics get brittle, beer gets skunky, sodium hypochlorite decomposes the list goes on and on. Most complex organic structures absorb radiation in the region of 200-300 millimicrons. All colored materials absorb radiation in the region of 400-800 millimicrons, their color being a function of their absorp- tion characteristics in this region. Generally speaking, all colored products also absorb in the region of 200-400 millimicrons. The theory of degradation of organic compounds by UV radiation can be stated briefly in over-simplified terms. Whenever an organic compound absorbs light energy it is raised to a higher energy level. In this excited state it may remain stable and transmit its energy to other molecules by collision it may re-emit the energy at a different wavelength from that which was absorbed (e.g., fluorescence) or it may decompose into a reactive anion or radical retaining the absorbed energy. It is very possible in a ULTRAVIOLET SPECTRA OF BENZOPHENONES I ///• BENZOPHENONE 4 0 / ! \ • 4HYDRC ß 2,5 • / 4 HYDROXYBENZO- 2,4 OlHYDROX BENZOPHE• zoo 300 400 WAVE:LENGTH IN MILLIMICRONS Figure 2.--Absorption spectra of benzophenones.
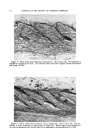
ULTRAVIOLET LIGHT ABSORBERS IN COSMETICS 361 mixture of products for one material to absorb radiation of a given wave- length, transmit this radiation energy selectively to the second material and, in so doing, revert to the original energy level. The second material may decompose or dissipate this energy, etc., as just described. This is the concept advanced to explain the catalytic effect of small amounts of carbonyl containing materials such as acetone and acetaldehyde on the photo decomposition of secondary butyl chloride by ultraviolet light in the 280 millimicron region. Secondary butyl chloride is completely transparent to light of these wavelengths, but the carbonyl group absorbs in this region (1, 2, 3). Much of the chemical work done studying the mechanisms of photo- chemical degradation has been done in the polymer field. However, the chemistry involved is universally applicable. Reactive intermediates formed by photochemical decomposition are free radical in nature and undergo reactions typical of free radicals. The degradative effects of ultraviolet light cannot be separated from that of oxygen. The normal mode of attack of oxygen on relatively inert materials, such as hydrocarbons, fatty acids, etc., is by way of a free radical mechanism. Oxygen is itself a free radical and readily combines with other free radicals to form peroxygen compounds. Therefore, oxidative STABILITY TO ULTRAVIOLET IO( 8( 6O 4O 20 0.6% IN 5MIL FILM - CELLULOSE ACETATE • A - U.EXPOSEO ---A & B -03 • B -- I000 HOURS-FADEOMETER -03 z -I'- _ -- -- 220 260 34)0 340 380 420 460 WAVELENGTH IN MILUMICRONS Figure 3.--Stability to ultraviolet light of 2,2'-dihydroxy-4,4'-dimethoxybenzo- phenone.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)