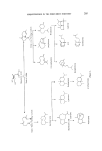
SESQUITERPENES IN THE PERFUMERY INDUSTRY 217 Longifolene A% 0 •r /•• ZnCIz //,• [solongifo!ene Figure 21. this ketone to the 7(a)H derivative is simple and this ketone is also of perfumery use as it possesses a sweet-woody odour. The rearrangement of isolongifolene epoxide has been reported (77-79) to yield two [3, T-unsaturated alcohols. No patents have been published on the rearrangement of the epoxide under most reaction conditions these alcohols are formed in less than 50•o yield, the main product is 8-oxo-7-[3- H-isolongifolane (Fig. 22). iso Longifolene-a -epoxide Figure 22. o 8- o xo -7 -/•-H-isolongifolane The sterochemistry of isolongifolene is a subject of academic discussion (76-79) and it is still not known beyond all doubt if the epoxide is, in fact, a- or [3. In the author's opinion, the a-structure is the more probable (80). Sodium dichromate oxidation of isolongifolene yields a complex mixture of ketones with a woody, vetiver type of odour. This mixture has found use in perfumery (81). Allylic oxidation of isolongifolene with tertiary butyl peracetate gives a mixture of 9a- and [3-acetoxy-isolongifolene. This has a woody, vertiveryl acetate odour and has found use in perfumery (82) (Fig. 23). Isolongi folene 9 Acetoxy - i solongi folene Figure 23.
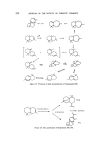
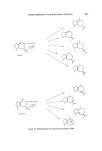
218 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The rapid commercial exploitation of longifolene in the last few years illustrates the interest shown by perfumers in new sesquiterpene products. With the considerable academic and commercial interest being shown in this hydrocarbon, further developments of interest to perfumers may well be expected. Sandalwood oil is much used in perfumery and has a large production its odour is too well known to be described in this paper. The main con- stituents of the oil are known (83) tz-santalene had a sweet-woody odour of excellent tenacity J3-santalene has a similar odour but is said to be less sweet than the tMsomer. tz-Santalol is considered to have the refined sweet- woody, tenacious sandalwood odour. Impure tz-santalol isolated by distil- lation from sandalwood oil is the santalol of commerce. J3-Santalol is said to have a similar odour but it occurs to a smaller extent in sandalwood oil and samples may have been contaminated with the tz-isomer. Perfumery opinion is that the tz-isomer is the preferred isomer (Fig. 24). (•-- Santa lene •- Santalene a- $antalol B--$antalol CH•OH Figure 24. The santalyl structure is related to the structure of 1ongifolene if it were possible to open the seven-membered ring of longifolene one would have a santalene structure. The sterochemistry is such that the opposite optical isomer to the natural santalene would be produced (Fig. 25).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)