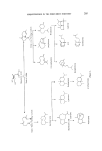
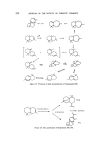
SESQUITERPENES IN THE PERFUMERY INDUSTRY 219 Figure 25. Sandalwood is a moderately expensive essential oil and its typical bouquet continues to be favoured by the perfumery industry. The search for sandalwood odours have therefore been intense. A number of chemicals with a sandalwood odour have been made. The so-called terpeno-phenols manufactured by the condensation of phenols with camphene followed by hydrogenation (84) have found application in a number of sandalwood bases. These have found a use in the industry but have not depressed the demand for the natural oil. The last addition to the sandalwood odours is Osyrol (and its homologs), and although this monoterpene is not a direct replacement for a-santalol, this speciality has a fine sandalwood odour and with its good performance in the middle notes it will be of wide interest in the formulation of perfume compounds where the sandalwood character is desired (85) (Fig. 26). Figure 26. The commercial synthesis of sesquiterpenes has yet to be achieved but much progress has been made in the academic synthesis of these compounds. Starting from ct-bromocamphor French workers were able to synthesize a- and 13-santalol in nine steps (86). The reaction sequence is too long for this synthesis to be of commercial interest. Figure 27.
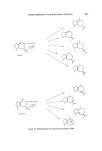
220 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Canadian workers have devised a route for the synthesis of camphere- none. This sesquiterpene is a valuable intermediate for the synthesis of a number of sesquiterpenes and in two steps it may be converted either to a-santalene or [I-santalene (87, 88) (Fig. 28). /•- Santalene i a-$antalene Figure 28. This synthesis might have been of commercial value but at the key cyclization stage two compounds are produced, one of which gives santalene and the other epi-santalene. An alternative approach was examined by American workers who synthesized 3-methyl-norcamphor from the Dieis-Alder product of methyl cyclopentadiene and ethylene. The construction and addition of the side chain to the camphene structure was more complex and again this synthesis does not offer, at present, any commercial possibility (89). A more direct synthesis has been described. The key step was the Dieis- Alder addition of geraniol to cyclopentadiene low yields at this stage appear to have discouraged exploitation of this path (90). The lactonization of camphene-8-carboxylic acid has been reported to yield a lactone with the correct arrangement of functional groups to be a santalene intermediate. Here again the key step produces not only the desired santalene structure but also the epi-santalene structure (91).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)