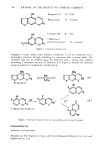
j. Soc. Cosmet. Chem., 30, 385-391 (December 1979) A simple nitrite assay method for the screening of raw materials commonly used in creams and lotions R. RAO GADDE and BHIKHU PATEL, [Vestwood Pharmaceuticals Inc., Buffalo, NY 14213. Received April 23, 1979. Synopsis A detailed colorimetric method for the determination of nitrite in raw materials adapting a modified Griess procedure is described. This simple method has been shown to be accurate, precise and applicable to about 80% of the raw materials used in pharmaceutical/cosmetic CREAMS and LOTIONS. The method is recommended for the RAPID SCREENING of RAW MATERIALS for NITRITE. INTRODUCTION Considerable attention, recently, has been given to nitrite analysis. The reasons are two-fold. First, nitrite is a precursor nitrosating agent to the formation of carcinogenic N-nitrosamines. Second, nitrite itself has recently been reported to be carcinogenic. Since the report by Fan et al. (1) about the presence of N-nitrosodiethanolamine in certain cosmetic lotions and shampoos, the cosmetic industry has been active in finding ways to minimize the level of N-nitrosodiethanolamine in cosmetic products. Since N-nitrosodiethanolamine is not added to products, it may be present either as an impurity in certain raw materials used in manufacturing the products or formed during the manufacture or storage. If a product contains both a nitrite and an amine (secondary or tertiary) there is a possibility of N-nitrosamine formation, but the rate of formation is controlled by many other factors such as pH, temperature, etc. Hence, minimizing nitrite content in products is an obvious approach to control N-nitro- samines in products. Nitrite is not added to any cosmetic products, but it is used as an anticorrosive agent in certain raw materials and also in certain drum seaming compounds. Furthermore, nitrite, along with nitrate, is ubiquitous in the environment it may therefore be present as an impurity in raw materials. In a N-nitrosamine control program, it is therefore desirable to screen raw materials for nitrite and eliminate problem raw materials with high nitrite content. The nitrite assay method which is used for raw material screening should be specific for nitrite as well as sensitive to nitrite levels at or below 1 mg/kg. Currently available nitrite assay methodology has been reviewed by Streuli and Averell (2). Methods based 385
386 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS on redox and acid-base reactions do not work satisfactorily at low levels of nitrite, and also these methods are not specific for nitrite. Colorimetric methods using diazotiza- tion and coupling reactions to produce an azo dye (commonly referred to as Greiss method, Bratton-Marshall method, etc.) have the desired specificity as well as the sensitivity. Numerous diazotizing agents and coupling agents have been reported to work satisfactorily (2). The method using sulfanilamide in the diazotization reaction and N-(1-naphthyl) ethylenediamine dihydrochloride in the coupling reaction has been studied extensively and it is the method recommended by the American Public Health Association for the quantitative determination of nitrite in water and waste water (3). The applicability of the colorimetric nitrite assay method, which uses sulfanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride, to raw materials used in cosmetic and pharmaceutical products (creams, lotions and shampoos) has been studied. Depending on the solubility of raw materials in water and chloroform, two separate sample preparation procedures have been developed. The two sample preparation procedures coupled with the sulfanilamide/N-(1-naphthyl) ethylenedi- amine dihydrochloride colorimetric nitrite assay method have exhibited desirable sensitivity, precision and accuracy. The results of these studies are reported in this communication. The details of the assay method are included for easy application of the method. The applicability and the limitations of the method are also discussed. EXPERIMENTAL MATERIALS A Schimadzu-Bausch and Lomb Model Spectronic-210 UV spectrophotometer was used to measure the absorbance of solutions. Sulfanilamide (assay 93% minimum) was obtained from Eastman Chemicals (Rochester, N.Y.). All other chemicals used were of ACS grade. Distilled water was obtained from a Barnstead all-glass still Model Fl. The nitrite stock solution (1.3 mg sodium nitrite/ml) was prepared, stabilized and standardized using standard procedures (3). Appropriate dilution of this stock solution was made just before use. A 1% solution of sulfanilamide [prepared in 10% (v/v) hydrochloric acid] and a 0.001% (w/v) aqueous solution of N-(1-naphthyl) ethylene- diamine dihydrochloride (NED) was used in the color development step of the nitrite assay procedure (3). SAMPLE PREPARATION PROCEDURES Nitrite in sample was extracted into water by following one of the two procedures A or B described below. Procedure A was applied to water soluble or miscible samples (e.g., propylene glycol, sodium lauryl sulfate, citric acid). Procedure B was applied to samples which were relatively more soluble in chloroform than in water (e.g., stearyl alcohol, glyceryl monostearate, lanolin oil). Samples which were not very soluble in either water or chloroform (e.g., TiO2) were analyzed by using Procedure A. In order to ensure minimum contamination or prevent loss of nitrite during sample preparation and assay, strict adherence to the following procedures is necessary. Procedure A A known amount of the sample (approximately ! g) was weighed into a 50-ml volumetric flask, added 40 ml of distilled water and shaken well to dissolve the sample.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)