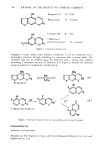
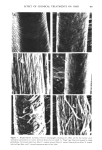
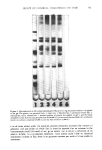
404 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS hair samples were suspended in 400 ml 6 mM sodium bicarbonate and rinsed with a stream of distilled water for I min prior to the tagging procedure. After tagging, unreacted iodoacetic acid was removed by rinsing in several changes of 95ø/3 ethanol, anhydrous ether and distilled water. Finally, the samples were air dried. Samples treated in this manner were fractionated into cuticle and cortex as described, and radioactivity was measured. Known weights of whole hair and cortex (1-5 mg) and lyophilized cuticle fraction (1 mg) were dispersed in 0.2 ml distilled water followed by 1 ml Soluene-350 (Packard Instrument Company, Downers Grove, Maine) at 55øC for 1.5 hr. The scintillation cocktail was 15 ml Dimilume-30 per vial (Packard Instrument Company, Inc.) and counting was performed on a Beckman Liquid Scintillation Counter Model LS-250 (Beckman Instruments, Fullerton, California). ELECTROPHORESIS PROCEDURES Samples of extracted, lyophilized protein were dissolved (1 mg/ml) in a solution containing 1% sodium dodecyl sulfate (SDS) (Bio Rad Laboratories, Richmond, California), 10 mM sodium phosphate (pH 7.0), 0.1 M dithiothreitol, 10% glycerol and 0.01% Bromophenol Blue. After inactivation for 5 rain in boiling water, the samples were applied directly to precast 10% SDS Bio-Phore polyacrylamide gels (Bio Rad Laboratories, California). Electrophoresis was performed for 2.5-3 hr at a constant current of 3 mA per tube (9). Gels were stained for 2 hr with 0.25% Coomassie Blue R-250 dissolved in isopropanol/glacial acetic acid/water (25/10/65 v/v) at 24øC. The destaining solution contained the same components (omitting Coomassie Blue) in volume ratios of 10/10/80, respectively. The gels were scanned with a Beckman Model 25 Spectrophotometer (Beckman Instruments, California) at a wavelength of 540 nm. RESULTS SCANNING ELECTRON MICROSCOPY (SEM) Virgin hair fibers were examined by SEM and these observations were then used to evaluate the surface morphology of fibers subjected to bleaching with H20 , reduction with ammonium thioglycolate (TGA), complete permanent waving and relaxation with sodium hydroxide. Analyses of the fibers indicated that very little change in surface structure could be attributed to these treatments except that relaxed hair showed more disordered cuticle than control samples (data not shown). The situation is quite different for fibers examined after dissolution of the cuticle by formic acid treatment followed by sonication (Figure 1). The conditions chosen for cuticle removal were standardized for virgin hair to give separation of cuticle and cortex so that no detectable damage to the cortical structure was observed. Identical conditions were then applied to the treated hair samples and the resulting cortical structures were observed under the SEM. Differences in the degree of cuticle separation and cortical damage depended on the original chemical treatment. In comparison to virgin fibers (Figure 1A), which showed virtually complete removal of cuticle from cortex (Figure lB), TGA-reduced fibers showed evidence of incomplete cuticle separation (Figure 1E). Permanent waved fibers showed an absence of cuticle remnants and extensiv6 damage to the cortex evident as
EFFECT OF CHEMICAL TREATMENTS ON HAIR 405 .? Figure 1. Representative scanning electron micrographs showing the effect of the hot formic acid- sonication procedures on cuticle removal from the hair shaft. A. Virgin hair fiber not treated by these procedures, B. treated virgin hair fiber, C. treated relaxed fiber, D. treated bleached hair fiber, E. treated reduced hair fiber, and F. treated permanent waved hair fiber.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)