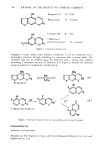
400 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS increasing the sample size to 10 g, the detection limit could be elevated to the level of 1 /zg/g. The recoveries of bergapten from several kinds of cosmetics are summarized in Table II, those at the spikage of 100 •g/g level being approximately 90%. CONCLUSION A procedure for the determination of bergapten in bergamot oil and cosmetics has been developed by use of the unfolding and closing reaction of the lactone ring of bergapten. The separated bergapten was quantitated gas chromatographically by a 5% SE-30/Chromosorb W 60-80 column using chrysene as an internal standard. ACKNOWLEDGEMENTS The authors wish to give their sincere thanks to Dr. Y. Ohta of the Institute of Food Chemistry (Osaka) and Dr. Sano of Research Institute for Wakan-Yaku of Toyama Medical and Pharmaceutical University for providing us with coumarin derivatives, and also to Dr. S. Kato of the National Institute of Hygienic Sciences (Tokyo) for providing us with chrysene standard. REFERENCES (1) U. R. Cieri, Characterization of steam nonvolatile residue of oil and some other essential oil, J. Ass. Oj•c. Anal. Chem., 52, 719-728 (July 1969). (2) F. N. Marzulli and H. Maibach, Perfume phototoxity, J. Soc. Cosmet. Chem., 21,695-715 (September 1970). (3) K. Mizuishi, M. Kazama, Y. Nakamura, T. Kan, H. Harada and T. Totani, Hygienic chemical studies on irritants (I)--Quantitative determination of bergapten in cosmetics, Annu. Rep. Tokyo &letr. Res. Lab. P. H., 26-1, 139-145 (1975). (4) P. j. Porcaro and P. Shubiak, Hazardous substances--Liquid chromatographic determination of bergapten content in treated or natural bergamot oils,J. Ass. O•c. Anal. Chem., 57, 145-147 (January 1974). (5) I. Kitagawa, H. Shibuya, H. Takeno, T. Nishino and I. Yosioka, Biogenetically patterned transforma- tion of eudesmanolide to elemophilanolide II--Structure of minor products obtained by acid treatment of 5o•, 6o•-epoxy-eudesman-8•, 12 olide, Chem. Pharm. Bull. (Tokyo), 24, 56-60 (January 1976). (6) S. A. Brown and J.P. Shyluck, Gas liquid chromatography of some naturally occurring coumarins, Anal Chem., 34, 1058-1061 (August 1962).
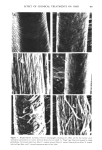
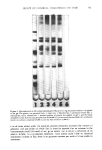
j. Soc. Cosmet. Chem., 30, 401-413 (December 1979) Comparison of the effects of some reactive chemicals on the proteins of whole hair, cuticle and cortex JOSEPH CHAO, A. EDWARD NEWSOM, IRENE M. WAINWRIGHT and ROGER A. MATHEWS, Biochemistry Laboratory, Redken Laboratories, lnc., 14721 Califa Street, Van Nuys, CA 91411. Received March 2, 1979. Presented at the Annual S•ientific Meeting, Society of Cosmetic Chemists, November 30, 1978, New York, New York. Synopsis COMPARISONS were made of the EFFECTS of four REACTIVE CHEMICAL processes on HAIR and on CUTICLE and CORTEX fractions isolated after chemical processing. The processes studied were bleaching, thioglycolate reduction, full permanent waving and alkaline relaxing. The comparisons were based on amino acid analysis, scanning electron microscopy, gel electrophoresis of extracted protein, and analysis of free sulfl•ydryl groups by alkylation with tritiated iodoacetic acid. Details of amino acid analyses of processed whole hair and of its cuticle and cortex are reported with differences shown among the several treatments. Cystine content was frequently altered by the chemical treatments and by alkali relaxing to the greatest extent of all. The radioactive tagging experiments showed that virgin hair contains an appreciable number of free sulfhydryl groups and that the treatmen. ts, except for bleaching, increase the number of such groups. The data suggest that sulfhydryl groups were about equally distributed between cuticle and cortex protein after thioglycolate reduction and complete permanent waving. More sulfhydryl was found in cuticle than in cortex, in bleached and in untreated hair. Alkaline relaxation resulted in greater production of -SH groups in cortex than cuticle. SEM shows the surface morphology to be not seriously altered by the chemical treatments studied. Upon post-treatment with hot HCOOH and sonication, however, differences in the degree of adhesion of the cuticle to the cortex became manifest, varying with the kind of process waved and alkali-relaxed hair showed clean cuticle separation from cortex but also exhibited some fraying of the cortical bundles. Disc gel electrophoresis of protein extracts from treated hair gave generally similar patterns except for alkali relaxed hair the latter exhibited virtually no separation of low molecular weight species due possibly to cross-linking reactions during treatment. Some preliminary work shows that formic acid treatment can alter the electrophoretic behavior of hair fractions speculatively due to a cross,.linking phenomenon. 401
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)