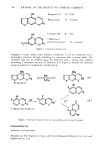
ANTI-IRRITANTS 423 intended for direct application to the skin. The irritant in this study was sodium lauryl sulfate (SLS). Dimer and trimer acids derived from linoleic acid, having the general cycloaliphatic ring structure: I-tOOC--(CH2)7-- ' CH NOH--CH2--CH--CH-•(CH2)7--COOH CHx x /CH--(CH2)4--CH3 (CH2) 4 --CH 3 have been the subject of many patents covering their use as mildness additives. Most of these patents--to Kelly and Ritter (12-18)--have been assigned to Cincinnati Milacron Inc. These patents cover the use of dimer and trimer acids, their simple esters and/or glycol esters, hydroxyl derivatives and amides. Dimer and trimer acids are available from Emery Industries Inc. and the Humko Chemical Division of Kraft. Diisopropyl dimerate and diisostearyl dimerate are available from Scher Chemical Co. Other dimer acid derivatives could presumably be made by other firms specializing in the production of esters and amides. The Kelly and Ritter patents specify that skin irritation is reduced "by adding small amounts of compounds having at least two polar groups (hydroxyl, carboxyl, amino, amido) separated by an organic radical of at least 15 carbon atoms, which contains a cyclic group." One of their patents (13)gives an excellent definition of anti-irritants: "This reduction or elimination of skin irritation occurs regardless of whether the mildness additive is applied to the skin prior to or simultaneously with the irritating chemical. Rinsing of the skin with water or a mild soap solution after application of the mildness additive, but prior to the application of the irritant, does not cause a significant change in the effect of the mildness additive when a skin irritant is subsequently applied." Among the examples quoted (18) are the 1:1 ratio use of Empol 1022 © (a linoleic acid dimer) with sodium lauryl sulfate or other alkyl benzene sulfonates to reduce average skin irritation ratings from +(7-9) to ratings as low as +(1-2). Exposure testing (immersion of guinea pigs to the thoracic region, in 45øC test solutions, 4« hours per day for three successive days) was carried out in extremely dilute (0.15%) solutions of various detergents by Kelly and Ritter (17). Skin damage to the animals was rated on a scale of 1-10, ranging from "extremely severe" (death of skin tissue rating = 1 +) to "moderate cracking" (rated 4 +) to "slight redness and edema" (rated 9+). On this (inverse) scale, addition of dimer acid mildness additives to 0.15% solutions of alkyl benzene sulfonate reduced their skin irritation ratings from 4 down to 9 (using 1:1 ratios of dimer acids). From 4 to 8 + using 1:1 ratios of dimer acid ester, and from 4 to 9 for dimer acid amides. In a separate series of experiments, sequential guinea pig immersion exposures to derreal irritants were run with the following results: (17)
424 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Sequential Guinea Pig Immersion in Alkyl Benzene Sulfonate Solutions Approx. Reduction Mildness Additive Average Rating of Irritation None 5.5 -- 0.15% TEA Dimer Soap 8.5 50% 0.15% Dimer diethanolamide 9.0 65% 0.15% Dimer acid 9.0 65% 0.15%PEG 400 dimerate 8.5 50% Kelly and Ritter believed (13) that the protection derived from such mildness additives results from their interaction with the keratin layer of the skin (blocking action) and not on interaction with the irritant. REDUCTION OF ALLERGENICITY Suskind and Majeti first published findings (19) of a so-called quenching mechanism whereby certain materials used as perfume vehicles (or found associated in nature with perfume sensitizers) apparently desensitize such materials. This paper was followed by a number of others (20-23) reporting on this interesting phenomenon. The major investigator, Donald L. J. Opdyke heads the Research Institute for Fragrance Materials (R.I.F.M.). Opdyke reported (20) three instances where individual aldehydes (phenylacetaldehyde, citral and cinnamic aidehyde) proved to be skin sensitizers when purified, yet did not induce sensitization reactions via the essential oil mixtures in which they occur naturally. Specifically, phenylacetaldehyde (a constituent of numerous citrus essential oils such as neroli, narcissus, magnolia, lily, rose, and tea oils), Citral (found in lemongrass oil and various other citrus peel oils) and Cinnamic aidehyde (identified in the essential oils of cinnamon leaves and bark, hyacinth, myrrh, patchouli and Bulgarian Rose) all show a curious common character- istic: the purified aidehyde sensitizes, whereas the "natural" mixtures do not. Yet surprisingly, one of these "natural mixtures," lemongrass oil, contains Citral at 75-85% levels! The balance consists of various terpenes which presumably have anti- sensitization properties. How can potent sensitizers be made innocuous by the addition of as little as 15-25% of other ingredients? Here indeed is an anti-irritant phenomenon of the first order! In discussing this quenching phenomenon at his 1977 Continuing Education Center lecture, Dr. Donald Opdyke pointed out that this phenomenon results primarily from the blockage of the initial induction of sensitization (by certain perfume aldehydic materials). Those who have previously been sensitized to (e.g.) Citral or Limonene may still react to them in such "natural" mixtures as lemongrass oil. Fisher and Dooms-Goosens discussed a different aspect of this problem. In their 1976 paper (23) they point out that during the aging process of a perfume, cinnamic aidehyde is oxidized to cinnamic acid. Perfume aldehydes may also react with alcohols in perfume mixtures, forming hemiacetals. In both cases, aidehyde-sensitive individuals generally no longer react to such aged perfume mixtures. Harry Saunders (Shaw
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)