NITRITE ASSAY METHOD 391 materials tested had no sample preparation problems affecting the determination of nitrite by this method (See Table IV). The interference with problem raw materials was found to be due to one of the following: gelling agents like methylcellulose do not permit the color development due to the gelling of the sample in water and materials like cellulose gum, and cocamide DEA and certain starches and polyethylene glycol derivatives have associated sample clarification problems. However an estimate of nitrite content in these materials could possibly be made if the nitrite nitrogen content is about 0.2 mg/kg or more in the sample. Certain dyes which absorb at 543 nm (e.g., F D & C Blue 1, D & C Green 5) cannot be assayed by this method. The method is not seriously affected by the acidic or basic nature of raw materials. However basic materials such as ammonium or sodium hydroxide may erroneously produce false low nitrite assay results. In such cases, it is preferable to adjust the pH of the sample solution prior to the addition of sulfanilamide solution with nitrite-free hydrochloric acid. CONCLUSION The nitrite assay method described was found to be very simple and useful for quick screening of raw materials for nitrite. The accuracy and precision of the method based upon the application to two raw materials were found to be excellent. Similar performance should be expected with most other raw materials. The method is very specific for nitrite since the color development step involves two reactions (diazotiza- tion and coupling) that individually and collectively reduce the number of possible interferences. ACKNOWLEDGEMENTS The authors express appreciation to Ms. Karen Burke and Mr. Roger Wright for their technical assistance and to Mr. Joseph Diliberto for his assistance in manuscript preparation. REFERENCES (1) T. Y. Fan, U. Goff, L. Song and D. H. Fine, N-Nitrosodiethanolamine in cosmetic lotions and shampoos, Food Cosmet. ToxicoL, 15,423-430 (1977). (2) C. A. Streuli and P. R. Averell, "The Analytical Chemistry of Nitrogen and its Compounds," Wiley-Interscience: New York, 1970 pp 117-144. (3) "Standard Methods for the Examination of Water and Waste Water, American Public Health Association: New York, 1976 pp 434-436.
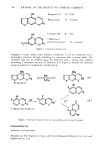
j. Soc. Cosmet. Chem., 30, 393-400 (December 1979) Detection and determination of bergapten in bergamot oil and in cosmetics HIDEYO SUZUKI, KEIZO NAKAMURA and MASAHIRO IWAIDA, National Institute of Hygienic Sciences, Osaka Branch, -43, I-chome, ttoenzaka, Higashi-ku, Osaka 540, Japan. Received July 27, 1978. Synopsis A reliable method for the EXTRACTION and DETERMINATION of BERGAPTEN has been developed. Treatment of bergapten with alkali solubilized the material in water through a lactone ring opening. Hydrophobic contaminants were then removed by washing with ether followed by lactone ring closure by heating with 10% sulfuric acid and the reformed bergapten extracted with chloroform. An aliquot of the solvent layer was determined using chrysene as an internal standard on a 5% SE-30 column by FID chromatography. A linear calibration curve was obtained for 15-1,500 ng of bergapten with a variation coefficient of 4.6% at the 33.3 ng level. A hundred/ag/g spike of bergapten in BERGAMOT OIL gave 92.6% recovery. The maximum bergapten content observed in seven commercial bergamot oils was !,o0o/.tg/g. INTRODUCTION It is well known that a bergamot oil which is widely used for cosmetic use causes photodermatitis under irradiation from sunlight or other UV sources. Bergamot oil generally contains several compounds of the coumarin family, i.e., bergapten (5- methoxypsoralen), citropten (limettin), bergamottin and 7-methoxy-5-geranoxycou- marin. Their structures are shown in Figure 1. Among them, bergapten is known to have the most harmful phototoxity (1,2). Analytical methods have been reported for bergapten in bergamot oil either by the use of gas chromatographic (3) or high-performance liquid chromatographic (HPLC) (4) techniques. These methods, however, have complicated clean-up procedures, distilla- tion, column chromatography, etc. In this report, the authors describe a simplified quantitative method for the determina- tion of bergapten in cosmetics, making use of unfolding and closing reactions of the lactone ring (5). Since almost all components of bergamot oil are of a hydrophobic nature, bergapten could easily be separated from the oil if it could be converted into another compound of a more hydrophilic nature. Although the o•, /5 unsaturated &lactone ring of 393
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)