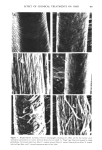
410 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The data for virgin and bleached hair show about a two-fold greater incorporation of tritiated iodoacetate in the cuticle than in the cortex. Both TGA-reduction and full permanent wave show about equal incorporation into cuticle and cortex, while hair relaxation shows a two-fold greater labeling in cortex than cuticle. SDS-POLYACRYLAMIDE GEL ELECTROPHORESIS Polyacrylamide disc gel electrophoresis was performed on protein extracted from hair which was processed by the four types of chemical treatments (Figure 2). In general, the virgin, bleached, permanent waved and reduced protein band patterns were similar. The 11-12 protein bands stained by Coomassie Blue can be grouped into roughly three sections of the gel. In the upper 3-4 cm of the gel there were three or four bands with molecular weights greater than 300,000 daltons. The second group of approximately six bands migrated between 3.5 cm and 6.5 cm from the top (in the 45,000-82,000 dalton molecular weight range). At about 8.5 cm from the top of the gel there were one or two protein bands of molecular weight about 27,000 daltons. The relaxer- treated hair proteins were probably crosslinked by interchain lanthionine and lysinoal- anine bonds so that electrophoretic separation was hindered. The cuticle and cortex fractions prepared by the formic acid-sonication method were also subjected to disc gel electrophoresis after protein extraction. The results of these experiments are not presented in detail since formic acid treatment apparently crosslinked a high percentage of the protein which remained at the top of the gel. To test the effect of formic acid on hair protein, we scraped cuticle cells from hair and extracted protein as before. The protein was then freeze-dried and divided into two equal aliquots. The first aliquot was dissolved in sample buffer and electrophoresed as a control. The second aliquot was treated with formic acid (88%) for 16 hr at 53øC and electrophoresed in parallel with the control (untreated) sample. The results show that formic acid does indeed distort the cuticle protein band patterns. Most of the formic acid-treated protein did not enter the gel which is suggestive of chemical crosslinking in these samples. The untreated sample of cuticle protein showed the presence of four lightly staining bands of high molecular weight and a predominant rapidly migrating component of molecular weight 21,000 to 24,000 dalton. This component was judged to represent 80 to 90% of the total cuticle protein by scanning the gels in a spectrophotometer. DISCUSSION The surface of hair was not materially altered by the chemical treatments studied, other than relaxers to a small extent however, SEM data show that the cohesion of the cuticle cells to the cortex fibers may be differentially affected by reactive cosmetic processes. Of the four types of chemical treatments, bleaching produced the least change in this property. Alkaline hair relaxation and the thioglycolate reduction step of the permanent waving process produced substantial changes in amino acid composition. Cystine levels were affected by the chemical treatments tested in TGA reduction and alkali relaxation, its direct oxidation to cysteic acid does not completely account for the cystine loss. In our present studies we found that chemical treatments could increase the concentra-
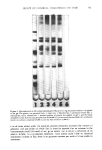
EFFECT OF CHEMICAL TREATMENTS ON HAIR 411 Figure 2. Electrophoresis on 10% polyacrylamide gels. Fifty •tl of a 1 mg/ml protein solution was applied to the gel. The protein was extracted from: 2. virgin hair, 3. bleached hair, 4. permanent waved hair, 5. reduced hair, and 6. relaxed hair. A standard mixture of protein was applied on gel •! and the bands identified in order from the top: phospharylase B (94,000 d.), bovine serum albumin (68,000 d.), ovalbumin (43,000 d.), carbonic anhydrase (29,000 d.), and soy bean inhibitor (21,000 d.). tion of some amino acids. For example, alkaline relaxation increased the content of glutamic acid and serine of whole hair. It must be stressed that an increase in the concentration (mol/1000 tool) of any given amino acid is simply a reflection of its relative stability to experimental treatment. Since amino acids labile to chemical treatments decrease in hair, there is an apparent increase per mole of those stable to treatment.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)