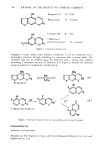
COMPARISON OF GUINEA PIG AND FETAL HOG SKIN 383 2.0 0.5 a Wavelength (cm-1) 3500 3000 3500 3000 2500 2000 1500 1000 500 Wavelength (cm '1 ) 2.0 0.5 Wavelength (cmd) 2.0 3500 3000 2500 2000 1500 1000 500 Wavelength (cm '1 ) Figure 6. FTIR spectra of a) human stratum corneum, b) guinea pig stratum corneum and c) fetal hog periderm. The permeability of human skin to many compounds is reported to be lower than other mammalian skins. Tregear (7) measured the permeability of skins to salts such as NaC1, KC1, NaBr, NaH2PO • and Na2HPO•, and ranked the species in order of increasing permeability: human, hog, guinea pig, rat and rabbit. Several other investigators have substantiated the low permeability of human skin and the similarity of human and hog skin. Bartek (8) showed that the permeability of human skin was closest to that of hog skin, followed by rat and rabbit skin, when he measured the diffusion of haloprogin, cordsone, testosterone and caffeine. Galey (9) showed good correlation between hog and human skin to tritiated water and Anisworth (10) did so for tri-butylphosphate. The low permeability of adult hog skin and its similarity to human skin support our conclusion that the permeability of fetal hog skin is high because the barrier is still undergoing development. Electron microscopy (performed by other investigators in this laboratory) confirmed the thinness of the stratum corneum layer of fetal hog skin, which was less than half the thickness of guinea pig skin. Formaldehyde pretreatment drastically reduced the permeability of fetal hog skin. This effect may be indicative of a more loosely organized structure. In addition, the absence of lipids in the fetal stage may contribute to the high permeability, although at 72 d gestation (term = 95 d) sebaceous glands which appeared to contain some functioning cells have been identified (11). Smith (12) reported only rudimentary sebaceous glands in newborn hogs. These sebaceous glands may not, therefore, be completely functional and the amount of surface lipids would be low.
384 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Amino acid analyses of the stratum corneum hydrolysates indicated more similarities than differences between species. However the relative amounts of ether- and water-extractable compounds (particularly free amino acids) showed large species differences. Guinea pig resembled human skin in this respect. Water-extractable materials are thought to be involved in water binding (13). In addition, the free amino acids, particularly the carboxylic groups, are believed to play a role in determining skin pH and to act as buffers (14). One of,the functions of skin lipids is to protect these free amino acids from being extracted by aqueous materials. Middleton (13) showed that these water soluble substances could not be removed by water extraction unless the stratum corneum had previously been delipidized. It is, therefore, not unexpected to find that fetal hog stratum corneum and periderm, which had low lipid contents, contain very small amounts of free amino acids and other water-extractable materials. REFERENCES (1) K. A. Holbrook and G. F. Odland, The fine structure of developing human epidermis: light, scanning and transmission electron microscopy of the periderm,J. Invest. Dermatd., 65, 16-32 (1975). (2) E.J. Singer, L.J. Vinson, W. R. Koehler, M.D. Lehman and T. Masurat, The nature of the epidermal barrier and some factors infiuendr•g skin permeability, Toxicol. Appl. PharmacoL Suppl. 2, 7, 7-19 (1965). (3) T. J. Franz, Percutaneous absorption: on the relevance of in vitro data, J. Invest. Dermatol., 64, 190-195 (1975). (4) S. Moore and W. H. Stein, Methods in Enzymology, Vol. 6, Academic Press: New York, N.Y., 1963 p 819. (5) H. P. Baden, L. A. Goldsmith and 13. Fleming, A comparative study of the physicochemical properties of human keratinized tissues, Acta Blochim. Biophys., 322, 269-278 (1973). (6) E.J. Singer, P. C. Wegmann, M.D. Lehman, M. S. Christensen and L.J. Vinson, Barrier development, ultrastructure, and sulfhydral content of the fetal epidermis,J. Soc. Cosmet. Chem., 22, 119-137 (1971). (7) R. T. Tregear, The permeability of mammalian skin to ions. J. Invest. Dermatol., 46:16-23 (1966). (8) M.J. Bartek, J. A. LaBudde and H. I. Maibach, Skin permeability in vivo: Comparison in rat, rabbit, pig and man,J. Invest. Dermatol., 58, 114-123 (1972). (9) W. R. Galey, H. K. Lonsdale and S. Nacht, The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water,.]'. Invest. Dermatol., 67, 713-717 (1976). (10) M. Ainsworth, Methods for measuring percutaneous absorption, J. Soc. Cosmet. Chem., 11, 69-78 (1960). (11) E. H. Fowler and M. L. Calhoan, The microscopic anatomy of developing fetal pig skin, Amer. J. Vet. Res., 25,156-164 (1964). (12) J. L. Smith, The microscopic anatomy of the integument of new born swine, M. S. Thesis, Michigan State University: East Lansing, Michigan, 1960. (13) J. D. Middleton, The mechanism of water binding in stratum corneum, Brit. J. Dermatol., 80, 437 (1968). (14) R. C. Burke, T. H. Lee and V. Buettner-Janusch, Free amino acids and water soluble peptides in stratum comeurn and skin surface film in human beings, YaleJ. Bid. Med., 38, 355 (1966).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)