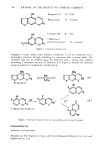
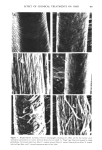
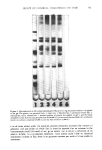
402 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS INTRODUCTION The effects of chemical treatments on hair proteins are quite complex. Many previous studies have concentrated on compositional changes in hair amino acids resulting from permanent waving, bleaching or straightening (for example, ref 1-4). In this paper, we compare the effects of four chemical treatments on hair from a single source using several methods. At the morphological level, direct comparisons of chemically treated hair by scanning electron microscopy (SEM) were made. At the molecular level, results of separation of hair proteins with polyacrylamide gel disc electrophoresis, of amino acid analysis of hair, and of radioactive tagging of free sulfhydryl groups of hair protein with tritiated iodoacetic acid are reported. Some experiments were performed on isolated cuticle and cortex fractions prepared by a formic acid-sonication procedure to determine the location of chemical effects on hair. MATERIALS AND METHODS HAIR SAMPLES AND CHEMICAL TREATMENTS Light brown virgin hair from one head was used throughout the study. Swatches were prepared from 4-in lengths of hair fibers cut from the proximal end of the hair. One end of the swatch was secured with dental wax. Each swatch weighed approximately 0.5 g. The swatches were washed for 5 rain consecutively in each of the following solutions: 95% ethanol, anhydrous ether and distilled water, and for 20 sec in 95% ethanol before drying between paper towels at 24øC. Two swatches were bleached with Kool Blue © Bleach (Redken Laboratories), containing 6% hydrogen peroxide, persulfate and ammonium hydroxide as major components. The swatches were placed on glass plates, saturated with bleach, and incubated at 40øC for 30 min. The swatches were then rinsed under a stream of distilled water for 5 rain and air dried. The permanent waving procedure (5) utilized the acidic permanent wave Trichoperm © 122N (Redken Laboratories). Reduction of hair samples was accomplished by saturating hair on rods with ammonium thioglycolate (pH 7.0) at 65øC for 24 min. The swatches were then rinsed under a stream of distilled water for 3 rain and dried at room temperature as before. These samples were designated as reduced hair samples. Two reduced swatches were saturated with neutralizing lotion (sodium bromate) for 5 rain before rinsing (5 rain) and finally air dry{ng after removal from the rods. These samples were designated permanent waved hair samples. Two swatches were treated with relaxer (2% sodium hydroxide in a cream base). Each swatch was combed through for 15 rain, with 42 g of relaxer, allowed to sit at 24øC for 5 rain and combed for another 2 min. Rinsing in distilled water for 5 rain and air-drying were performed as before. AMINO ACID ANALYSIS Acid hydrolysis and chromatography on a Beckman 121M Amino Acid Analyzer were performed as detailed earlier (1,5). The amount of L-plus-meso-lanthionine was
EFFECT OF CHEMICAL TREATMENTS ON HAIR 403 calculated using the assumption that the material in the two peaks had the same ninhydrin color constant (Kf value). PROTEIN EXTRACTION The procedure for protein extraction was modified after Baden et al. (6). Individual samples of whole hair, cortex and cuticle (about 100 rag) were extracted in a degassed reducing solution (50 ml for whole hair and cortex samples and 20 ml for cuticle) consisting of 0.2 M Tris buffer, 6 M urea and 0.2 M mercaptoethanol at pH 9.2 and 50øC for 1.5 hr. Homogenization was accomplished with a Polytron Homogenizer (Brinkmann Instruments, New York and Ontario, Canada) for 20 min at 4øC. The solubilized protein was recovered in the supernatant after centrifugation at 28,000 g for 30 min (BeckmanJ-21B Centrifuge, Fullerton, California). The reduced disulfide bonds were reacted with 3 g solid iodoacetic acid (7). The procedure was carried out at pH 8, with stirring in a foil-covered reaction vessel, for 1.5 hr. The solution was then dialyzed overnight against distilled water (two changes) and lyophilized (Virtis Model 10-100-V, New York). The dry powders were stored at 4øC. The radioactively tagged hair samples (to be described later) were treated in the same way except that the thiol groups formed during the extraction procedure were aminoethylated using 3 ml ethylenimine per sample, according to the procedure of Cole (8). SEPARATION OF CUTICLE AND CORTEX Approximately 100 mg samples of whole hair .were refiuxed with 88% formic acid at 80øC for 1 hr. After cooling to room temperature, the samples were shaken for 16 hr in a gyratory shaker. Sonication was performed after increasing the volume to 40 ml with 88% formic acid. The sonicator (Lab-Line Instruments, Inc., Illinois) was run at 80 watts for 40 min with the sample in a water-cooled container. The conditions were established by testing virgin hair with the procedure and verifying cuticle removal by light microscopy. Cortical fibers were removed from each flask and air dried. The remaining solutions were dialyzed against distilled water for 48 hr and lyophilized. MICROSCOPIC TECHNIQUES Scanning electron microscopy was performed as described earlier (5), except that the samples were affixed to SEM stubs with Walsco Tube Kote. RADIOACTIVE TAGGING WITH 3H-IODOACETIC ACID The reaction solution (250 ml total volume) contained 0.2 M Tris buffer at pH 8.0 and 1.7 mM sodium (ethlenedinitrilo)-tetraacetic acid. The hair sample (approximately 200 mg) was placed in a reaction vessel and a pH-stat (Brinkmann Instruments, Model E526, Westbury, New York) was attached so that a constant pH 8 was maintained. Iodoacetic acid (100 mg dissolved in 0.5N NaOH) together with 1 mCi 3H-iodoacetic acid was added slowly with stirring. Incubation was for 2 hr. The bleached and permed
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)