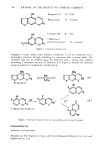
396 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS [ Et20 I H20 I H20 Sample (Contg. 10-100/•g of bergapten) 10% methanolic KOH 5 ml reflux with stirring 3 hrs NaCl saturated H20 40 ml wash with Et20 20 ml x 2 H20 10% H2SO 4 20 ml heat on water bath 1 hr extract with CHCI• 30 ml x 3 CHCI 3 wash with 1% NaHCO• 20 ml x 2 and H20 20 ml CHCI• xylene 1 ml concentrate with K-D concentrator chrysene 300/•g and acetone 3 ml GLC Step 1 Step 2 Step 3 Scheme 1. Analytical procedure for bergapten. chloride solutions, the mixture was transfered to a separatory funnel and extracted twice with 20 ml of ether, discarding the ether layers. The water layer was neutralized with sulfuric acid to which an additional 20 ml of 10% sulfuric acid was added. After heating for 1 hr on a water bath, the mixture was extracted three times with 20 ml of chloroform. Step 2. The chloroform layers were combined, washed twice with 20 ml of 1% sodium bicarbonate solution, then washed with 20 ml of water and dried over anhydrous sodium sulfate. One milliliter of xylene was added to the dried material which was then concentrated to a volume of 1 ml using a Kuderna-Danish concentrator. Step 3. One ml of chrysene standard solution was added to the concentrate, and then made up to 3 ml with acetone. Five/•1 of the prepared solution was injected into the gaschromatograph. The bergapten content in the sample was estimated with the aid of the calibration curve. PREPARATION OF THE CALIBRATION CURVE To 1 ml of bergapten standard solution were added 1 ml of chrysene standard and 1 ml of acetone. Five M1 of this solution was injected into a gas chromatograph. The relative peak heights of bergapten to chrysene were estimated against the bergapten content per tube.
DETECTION OF BERGAPTEN 397 RESULTS AND DISCUSSION STEP 1 In order to set up a suitable condition for the unfolding of the lactone ring in bergapten, the relationship between refiuxing time and the recoveries of bergapten was evaluated both with the presence and with the absence of bergamot oil. One milligram of bergapten was hydrolysed in 10% methanolic potassium hydroxide for 0.25-3.5 hr, followed by the ring closing reaction in 10% sulfuric acid and the procedures described below in Step 2 and 3. The results are shown in Figure 3-1. The unfolding ratio of the lactone ring of standard (4) 0 1 2 3 (hr) Reoe1ux %ime 1. Ring unoeolding reaction O O Bergapten 1 m R in 10• He0H-KOH • BerRapten 1 m R and berRamot oil 1R ñn 104 HeOH-KOH (4) i i , $ 0 0.5 1.0 1.5 (hr) Heating time 2. Ring closing reaction Bergapten 1 mg in 10• H2SO 4 Figure 3. Reaction times of ring unfolding and closing in Step 1. bergapten after 1, 2, 3 and 3.5 hr were 87.9, 90.9, 92.0 and 92.0% respectively. The velocity of the hydrolytic unfolding was a little slower in the presence of bergamot oil, but was found to reach the maximal level after 3 hr. Further reflux reactions were run, therefore, for 3 hr. The effect of heating time with 10% sulfuric acid on the recoveries of closed ring bergapten was investigated the results are indicated in Figure 2-2. It was confirmed that recovery was maximal at 92% at after 1 hr. Thereafter all further experiments were heated for 1 hr. STEP 2 Bergamot oil samples that had undergone the procedures in Step 1 were found to show disturbing peaks on the gas chromatograph, which might be attributed to the partial hydrolysis of the sample during the procedure producing various kinds of free fatty acids (see Figure 4-1). Such acids can easily be removed by washing with alkaline
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)