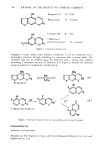
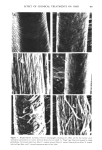
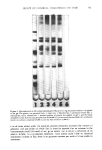
412 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The data on the effect of chemical treatments on amino acid composition and labeling of free thiol groups with tritiated iodoacetic acid is in general agreement with previously published work (1-4). The use of one homogeneous hair source, as in this work, is advantageous in eliminating the possibility of differences in reactivity due to differences in amino acid composition (14), and in free sulfhydryl content (15). Although the use of a single hair sample may not be representative of the overall population, it is useful in facilitating comparisons of different chemical treatments. Amino acid degradation may have been produced by formic treatment used in some of the methods employed. Solutions of the free amino acids (125 mmol/ml) histidine, threonine, glutamic acid, leucine, cysteine and especially serine were shown to be unstable to formic acid (24 hr at 110øC). The measurement of stability of free amino acids to hot formic acid does not necessarily reflect their stability in the polymerized state (peptides). Since we do recover large amounts of serine (for example) from formic acid treated hair, we must assume that the intact protein stucture produces a protective or sparing effect on bound serine. It is of importance to note, however, that our data on the amino acid composition of virgin cuticle isolated by formic acid-sonication generally resembles that of other workers using less severe methods of cuticle removal (10,11). The mechanism of action of formate on hair protein is not well characterized in our studies. Electrophoresis of cuticle protein (prepared by scraping the cuticle instead of formic acid-sonication treatment) suggested that formic acid crosslinked cuticle protein so that it did not enter the 10% gels. The untreated control cuticle protein preparation showed no such effect. A component of cuticle protein, perhaps a major subunit of the cuticle structure, of molecular weight about 21,000 to 24,000 daltons, was evident in the absence of formic acid treatment. This implies that the cuticle may be composed of small molecular weight repeating subunits that are aggregated into larger structural components. Other investigations have shown that formic acid can formylate oxygen and nitrogen on amino acid side chains (16,17). The mechanism by which formic acid crosslinks or promotes the crosslinking of cuticle protein is at present unknown. We speculate that formate can produce highly reactive free radicals on amino acid side chains which crosslink adjacent protein chains. This possibility must await experimental verifica- tion. ACKNOWLEDGEMENTS We thank Dr. Ronald Harris for his support. We appreciate the contributions made by Holly West (scanning electron microscopy) and Robert Bono (initiation of the project and preparation of the radioactively tagged hair samples). George Lefeber and Lois Blanchard are acknowledged for the photography, and Ava Baffoni for the typing. REFERENCES (1) A. E. Newsom and P. Shapshak, The use of two programs on the amino acid analyzer for the separation of non-protein compounds and protein amino acids in cosmetically treated hair, unpublished.
EFFECT OF CHEMICAL TREATMENTS ON HAIR 413 (2) J. G. Gumprecht, K. Patel and R. P. Bono, Effectiveness of the reduction and oxidation steps in acid and alkaline permanent waving,J. Soc. Cosmet. Chem., 28, 717-732 (1977). (3) L.J. Wolfram, K. Hall and I. Hui, The mechanism of hair bleaching, J. Soc. Cosmet. Chem., 21, 875-900 (1970). (4) P. Bore, J, Arnaud and G. Kalopissis, Novel process for improving and modifying the properties of hair, U.S. Patent 3,908,672 (1975). (5) R. P. Bono, The use of a cuticle dissolution technique to ascertain the sites of action of cosmetic reactive processes in human hair, J. Soc. Cosmet. Chem., (in press). (6) H. P. Baden, L. D. Lee and J. Kubilus, A genetic electrophoretic variant of human hair polypeptides, /lmer. J. Hum. Genet., 27,472-477 (1975). (7) C. H. W. Hits, Reduction and S-carboxymethylation of proteins, in C. H. W. Hirs, "Methods in Enzymology," Vol XI, Academic Press: New York, London, 1967 pp 199-203. (8) R. D. Cole, S-Aminoethylation, in C. H. W. Hirs, "Methods in Enzymology," Vol XI, Academic Press: New York, London, 1967 pp 315-317. (9) SDS-Biophore gel electrophoresis instruction manual, Bio Rad Laboratories Bulletin Number 4408 (1978). (10) J. A. Swift and B. Bews, The chemistry of human hair cuticle I: A new method for the physical isolation of cuticle,J. Soc. Cosmet. Chem., 25, 13-22 (1974). (11) J. H. Bradbury, G. V. Chapman, N. L. R. King and J. M. O'Shea, Keratin fibers III: Amino acid analyses of histological components, •4ust. J. Biol. Sci., 23,637-643 (1970). (12) L.J. Wolfram and M. O. Lindemann, Some observations on the hair cuticle,J. Soc. Cosmet. Chem., 22, 839-850 (1971). (13) K. L. Ziegler, New cross-links in alkali-treated wool,J. Biol. Chem., 239, 2713-2714 (1964). (14) J. M. Gillespie and M.J. Frenkel, The diversity of keratins, Comp. Biochem. Physiol, 47B, 339-346 (1974). (i5) J. M. Gillespie and R, C. Marshall, The proteins of human hair and nail, Tenth I.F.S.C.C. Congress, Australia, 3, 559-573 (1978). (16) L. Josefsson, Studies with model substances on the mechanism of the formic acid-induced reversible inactivation of protein enzymes, Biochim. Biophys. •4cta, 115,148-159 (1966). (17) S. U. Lakshim and L. K. Ramachandran, Formylation and deformylation of lysozyme and papain, Ind. J. Biochem. Biophys., 11, 17-21 (1974).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)