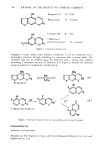
408 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Amino Acid Analysis of the Cortex Fraction. (mol/1000 mol total amino acids analyzed) Virgin Bleached Permanent Reduced Relaxed Amino Acid Hair Hair Waved Hair Hair Hair Alanine 45.2 + 1.8 46.5 + 0.8 45.9 _+ 0.1 46.6 + 0.4 47.9 + 0.1 Valine 53.8 -+ 4.6 51.8 + 0.8 57.3 + 1.2 57.8 +_ 1.0 59.2 _+ 0.6 Leucine 65.4 _+ 0.2 66.9 _+ 0.4 66.8 +_ 0.1 67.6 + 1.1 70.2 + 0.3 Isoleucine 26.2 +_ 1.7 26.4 +_ 0.4 27.7 +_ 0.1 27.6 +_ 0.5 28.8 +_ 0.2 Alloisoleucine Trace n.d. 2.0 + 0.1 n.d. 10.8 +_ 0.1 Proline 81.9 -+ 0.2 76.3 + 0.6 82.0 + 0.7 99.5 + 3.2 90.7 _+ 0.2 Phenylalanine 16.5 + 1.0 17.1 + 0.1 16.3 + 0.0 16.6 + 0.3 17.3 + 0.0 Methionine 5.4 + 2.2 4.2 + 0.1 5.1 + 0.1 5.3 + 0.2 5.4 _+ 0.1 Glycine 54.7 + 3.6 54.4 + 1.5 55.4 _+ 0.2 59.0 + 0.7 57.6 + 0.1 Serine 108.0 + 3.4 108.3 + 0.1 108.5 + 0.6 104.4 + 0.2 114.8 + 0.9 Threonine 77.3 + 0.6 76.8 + 0.1 78.9 + 0.0 77.7 + 0.1 84.3 + 0.5 Tyrosine 21.5 + 0.0 21.6 + 0.1 20.9 + 0.0 21.8 + 0.3 23.8 + 0.3 Aspartic Acid 56.8 + 1.5 59.1 + 0.2 57.5 + 0.1 58.0 + 1.1 59.9 + 0.1 Glutamic Acid 131.7 + 1.2 132.4 + 0.0 131.2 + 0.3 133.2 + 1.9 139.6 + 0.4 Lysine 25.3 + 0.8 25.8 + 1.3 25.7 + 4.6 24.5 + 2.2 20.7 + 0.3 Arginine 70.1 + 2.2 70.0 + 3.0 57.9 + 1.0 69.1 + 6.5 62.6 + 0.9 Histidine 9.2 + 0.4 9.1 + 0.4 7.6 + 0.1 9.0 + 1.0 8.1 + 0.2 Cysteic Acid Trace 21.7 +_ 0.1 5.7 +_ 0.3 Trace 1.5 +_ 0.1 Half-cystine 150.6 + 4.3 132.2 + 0.1 140.8 + 0.6 116.7 + 0.8 73.8 + 1.9 Lysinoalanine n.d. n.d. n.d. n.d. 3.7 + 0.1 L-+-meso- n.d. n.d. n.d. 6.0 + 0.1 30.6 + 0.5 lanthionine Carboxymethyl- n.d. n.d. 7.5 + 0.0 6.6 + 0.5 n.d. thio-cysteine n.d.--not detectable. Values given +_ the standard deviation with n = 2. It is evident from the data and other studies that cystine is most often altered in such chemical reactions reduction and relaxation exhibit the largest change in cystine. Insight into the kind of hair modification induced by reactive treatment is gained by analysis for formation of new compounds in the untreated hair. Cysteic acid, an oxidation product of the amino acid cystine, was virtually absent in untreated hair but was increased greatly by permanent waving and bleaching (Table I), i.e., where oxidants were employed in processing. Reduction and relaxation of hair also induced formation of small amounts of cysteic acid and increases in the quantities of alloisoleucine and lanthionine. Lysinoalanine was detected predominantly after alka- line hair relaxation. The *'mixed disulfide" of cystine and TGA (carboxymethylthiocys- teine) was found in reduced and especially in permanent waved hair, in accordance with previous observations (2). RADIOACTIVE TAGGING OF FREE THIOL GROUPS IN VIRGIN AND CHEMICALLY TREATED HAIR Since the reactions seemed to affect the cystine content of hair, it was of interest to measure the concentration of thiol groups in virgin hair and to compare the result to the number of thiol groups formed during alteration of disulfide linkages in hair by reactive treatment.
EFFECT OF CHEMICAL TREATMENTS ON HAIR 409 Free thiol groups were quantitated by reacting them with the tritiated carboxymeth- ylating agent iodoacetic acid. The carboxymethylation reaction is pH dependent and under the conditions of our analysis was specific for the thiol groups of cysteine. Therefore, the number of moles of iodoacetic acid bound to hair in the reaction is proportional to the number of free thiol groups available for carboxymethylation. Table IV shows the effect of the treatment reactions on the subsequent incorporation Table IV Incorporation of [3H]-iodoacetic Acid into Whole Hair After Various Reactive Chemical Treatments. Treatment /z mole/g • Untreated 2.46 Bleached 0.30 Reduced 506.50 Permanent Waved 64.58 Relaxed 70.35 •Data given in/2 moles 3H-iodoacetic acid bound per g hair. of tritiated iodoacetic acid into whole hair. The control hair contains an appreciable content of free sulfhydryl groups which are available to react with iodoacetic acid. From the specific activity of the tritiated iodoacetic acid used to tag reactive sulfhydryl groups (290.8 mCi/mmol) in hair, we calculate that virgin hair contains approximately 2.46 m3t mole of sulfhydryl per mg (assuming that 1 mol of sulfhydryl reacts with 1 mol of 3H-iodoacetic acid). Bleaching lowers the number of the free sulfhydryl groups reactive with iodoacetic acid by 88%. As one would expect thioglycolate reduction of hair produces a 200-fold increase in iodoacetic acid incorporation subsequent to TGA treatment. Sodium bromate oxidation of TGA-reduced hair resulted in an 87% reoxidation of the sulfhydryl groups to disulfide bonds as indicated by the decrease in tritiated iodoacetic acid reactivity in the reoxidized samples. Relaxed hair incorporated about the same amount of radioactively labeled iodoacetic acid as permed hair indicating an equal production of free sulfhydryl groups by these treatments. In an attempt to locate the morphological site(s) of action of the reactions iodoacetic acid tagged hair samples were treated with formic acid and then sonicated to separate cuticle from cortex. Table V shows the incorporation of labeled iodoacetic acid into the cortex and cuticle fractions. Table V Incorporation of [•H]-iodoacetic Acid into Cortex and Cuticle s after Various Chemical Treatments. Treatment Fraction /z mole/g 2 Virgin Cuticle 2.68 Cortex 1.18 Bleached Cuticle 0.36 Cortex 0.22 Reduced Cuticle 318.97 Cortex 317.13 Permanent Cuticle 73.60 Waved Cortex 68.17 Relaxed Cuticle 33,77 Cortex 72.11 'Weights of cuticle were calculated by subtracting the weight of cortex from that of whole hair. 2Data given in/z mole 3H-iodoacetic acid bound per g hair.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)