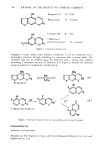
j. Soc. Cosmet. Chem., 30, 415-427 (December 1979) Anti-irritants ROBERT L. GOLDEMBERG, Rakuma Laboratories Inc., P.O. Box 2083, South Hackensack, NJ 07606. Received June 7, 1979. Synopsis ANTI-IRRITANTS are best categorized via three potential mechanisms of action: chemical COMPLEXATION of the irritant, BLOCKING of keratin reactive sites, and reduction of physical contact. Examples of each of these three mechanisms are given. STINGING phenomena are then examined, as well as other EYE IRRITATION problems. Specificity of the anti-irritancy of various ingredients (plus their efficacy) is also explored. Reduction of allergenicity to perfume ingredients and lanolin is also considered. INTRODUCTION In the early 1960's it began to be evident that there was more than one way to formulate safe topical preparations. The classic approach until then had always been rather straight forward: simply withhold any ingredients which may prove irritating in your product. This practice, however, frequently deprived formulators of valuable materials which had excellent functional properties, or which provided desirable esthetic benefits. We gradually began to realize that it was possible to add ingredients to topical formulations to reduce their eye sting, their primary skin irritation, and even to lessen the likelihood of allergic reactions. It has taken approximately 15 years for the state of the art to advance to this point. Patents now issue rather routinely to cosmetic and pharmaceutical manufacturers, claiming anti-irritant properties for various formula additives. It has been an exciting 15 years for those engaged in such studies. As part of two major reviews (1,2) concerning such anti-irritancy phenomena, the author previously postulated three mechanisms' by which anti-irritants may function: (1) via complexation of the irritant itself (2) by blocking otherwise chemically reactive sites of skin keratin--such as the cysteine residues resulting from UV or other degradation of cystine cross-linkages and (3) by reducing the degree of physical contact with the skin. 415
416 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Although occasionally effective, physical prophylaxis is not of great interest today. This paper will therefore concern itself primarily with chemical means of combating topical irritancy. CHEMICAL COMPLEXATION OF IRRITANTS This mechanism for inactivating irritants is well illustrated by the use of amphoteric imidazoline surfactants, which can totally eliminate harsh eye irritation effects such as those resulting from menthol. In one series of experiments which we ran (Table I), a Table I Mentholated Alcoholic Product (Pre-Electric Shave) Draize Eye Irritation Scores (3 rabbits No rinse-out) 24 hr 48 hr 72 hr Control (no Menthol) Control plus 0.70% Menthol Control plus 0.70% Menthol plus 0.14% Amphoteric 2. 10.0 6.2 5.0 14.7 12.4 6.5 0 0 0 pre-electric shave formulation (20% butyl stearate in ethanol) produced "base line" Draize eye irritation scores of 10.0, with no menthol. Addition of 0.7% menthol increased these initial scores to 14.7. Further addition of 0.14% Miranol C2M © (Amphoteric 2) to this formula reduced Draize eye irritation scores to zero. Perhaps more significant, such "mentholated" formulas could literally be "titrated" down from Table II Cologne Eye Irritation Draize Scores (3 Rabbits No Rinse-Out) 72-Hour Scores 7-Day Cumulative Cornea Total Cornea Total Only Score Only Score FORMULATION Control Cologne • 8 17 34 80 Control + 2% Polypropylene Glycol P2000 0 4 0 28 Control + 0.1% Azulene 0 5 0 28 Control + 0.14% Miranol C2M © 0 6 0 29 Control + 0.5% Polypropylene Glycol P2000 0 8 0 39 Control q- 0.3% PVP-K30 0 9 0 56 Control + 0.3% Thiodiglycolic Acid 5 11 13 44 Control + 0.3% Miranol 2MCA © 7 17 27 92 *The control cologne consisted of.' 2.5% Fragrance 1.5% Propylene Glycol 96.0% SDA 39C (which itself contains 1% Diethyl Phthalate) 100.0%
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)