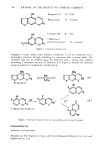
ANTI-IRRITANTS 425 Mudge Co.) provided a somewhat different view (24) of this difference in individual reactivities to fresh vs. aged fragrance compounds. He raised the possibility that polymerization takes place, resulting in differing skin residence times due to changed evaporation rates. Finally, Clark, Cronin and Williamson noted (25) that allergenicity to lanolin appears to derive from its natural free fatty alcohols, and that hypersensitivity to lanolin is apparently enhanced in the presence of ethoxylated alkylphenol surfactants. An interesting sidelight on cosmetic use of protein preparations was the work by Schuster (9) demonstrating that protein cococondensates of molecular weight 750 show less than one-fifth the eye irritation of slightly shorter chain lengths (mw 500). Further, rabbit eye irritation tests on blends of such protein cococondensates with sodium lauryl ether sulfate show synergistic mildness effects, reducing the eye irritation by one-third. Since we already know (from other studies) that the degree of ethoxylation is crucial to the irritation produced by SLES, this raises the possibility of formulating almost totally non-irritating shampoos, via combination of 3-7 mol ethoxylated alkyl sulfates with 750 mw protein condensates. Verdicchio and Waits, in a patent assigned to Johnson & Johnson (26) disclose high-foam betaine-based shampoo formU•lations having low occular irritation. They believed that reduction of irritation results when a complex forms between equi-molar quantities of amphoteric betaine surfactants and anionic surfactants such as alkyl sulfates. They then added nonionic POE Sorbitan esters to further reduce irritation, the preferred ratio being 1:1:3 (betaine to anionic to nonionic). Examples include the following two formulations: Carboxy betaine Tridecyl alcohol ether sulfate POE 20 SorbitanMonolaurate Water, dyes, preservatives, etc. Tegobetaine C © (31%) Tridecyl alcohol ether sulfate (65%) POE 44 Sorbitan Monolaurate Water, thickener, dye, etc. 15.0 15.0 12.6 qs 100.0% 17.1 8.3 15.0 qs 100.0% SUMMARY PROCEDURE There is now a considerable body of information available on the use of various materials to moderate the irritation potential of topical formulations. A few original papers have been published, and many patents have been issued. Much information is also available from raw materials suppliers anxious to show the beneficial value of their products. When the formulator is faced with the fact that his consumer product is irritating, he
426 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS should of course first attempt to remove (or reduce the level of) obvious offenders in the formula. If the product is then still too irritating, the next step is to review the literature and put together a list of potential anti-irritant additives which appear applicable in this product--in terms of probable compatibility, cost, skin feel, etc. The most likely anti-irritant candidates are then entered (singly) at appropriate levels and the usual routine checks made of any apparent formulation changes: stability, package or perfume compatability, effect on various esthetic qualities, on foaming, or whatever else is important to the success of that particular product. Failures at this level are discarded. The surviving formulas are put through a screening set of animal studies--usually with only a few animals. The screen may even be limited to those particular tests which previously showed the original product to be too irritating. The original formulation (without additive) must always be run simultaneously, as a control. Such controls are necessary, since the condition of the test animals may be judged by different personnel, or the animals themselves obtained from a different source than those on whom the product was originally tested. Always run a known control--from the same product batch as originally tested if at all possible. Comparison of irritation tests carried out at different times, at different test facilities, or from different batches of product is essentially meaningless. When the irritation reports come in, one has to judge which are significant, in view of the fact that this was only a limited screening study and therefore that a meaningful statistical analysis is impossible. The animal testing lab can often be useful at this stage. Discuss their reports with them. They actually observed the behavior of the animals. It is not wise to depend solely on written reports in such matters. Finally, in our own experience, we have frequently noted that anti-irritant effects are additive. If two ingredients each appear helpful individually, it is worth running further tests trying them in combination. REFERENCES (1) R. L. Goldemberg, Use of anti-irritants in cosmetic formulating, J. Soc. Cosmet. Chem., 16, 317-340 (1965). (2) R. L. Goldemberg and L. Safrin, Reduction of topical irritation, J. Soc. Cosmet. Chem., 28, 667-679 (1977). (3) J. A. Faucher, E. D. Goddard and R. B. Harriman, Protection of the skin by a cationic cellulose polymer, Cosmetics & Toiletries, 92, 39-44 (1977). (4) T. Getsrein, Non-irritating shampoo compositions containing stearyl amine oxide, U.S. Patent 4,033,895 (1977). (5) H. F. Drew andJ. G. Voss, Can. Patent 639,398 (1962). (6) S. G. Wilier, P. R. Yust and R. Kelly, Method of inhibiting skin irritation, U.S. Patent 4,076,799 (1978). (7) H. E. Reich, personal communication (1978). (8) I. V. Nosar, V. A. Drashchinskaya, B. S. Kolomiets and V. I. Tsesarskaya, Shampoo for children, USSR Patent 578,965 (1977). (9) V. L. Johnson, Innovation in protein products and technology, Cosmetics & Toiletties, 92, 29-36 (1977). (10) J. F. Gerecht, Aqueous cosmetic composition containing amine oxides, U.S. Patent 4,048,338 (1977). (11) J. N. Masci and N. A. Poirier, Detergent composition, U.S. Patent 3,055,836 (1962). (12) R. Kelly and E.J. Ritter, Mildness additive, U.S. Patent 3,538,009 (1970).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)