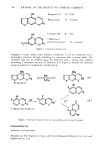
394 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS OR Bergapten (I) R = CH• Bergamottin R = Geranyl OR H3CO • Citropten (II) R = CH 3 7-Methoxy-5- geranoxycoumarin R = Geranyl Figure 1. Coumarins in bergamot oil. bergapten is quite stable under ordinary conditions, it can be converted into a hydrophilic structure through unfolding by treatment with a strong alkali. The unfolded ring can be refolded again by treatment with a strong acid, without provoking a secondary reaction (cf. Reaction B in Figure 2) because the hydroxyl group at position-5 is masked by a methyl group. Bergapten KOH H2S04 OH O- R• 0 H- R• 0 O0 O. CH 3 ' %•C00- ( A ) 5-Hydroxycoumarin OH Figure 2. Reaction mechanism of lactone ring unfolding and closing of bergapten. (B) EXPERIMENTAL MATERIALS AND REAGENTS Bergamot oil. The bergamot oil was a gift from Kobayashi Perfumery Co., Ltd., and Ogawa and Co., Ltd.
DETECTION OF BERGAPTEN 395 Bergapten. Bergapten was isolated from the bergamot oil according to the following procedure. Thirty g of Florisil © (Wako Pure Chemical Industry, Ltd., Osaka, Japan 535-02585) (60-100 mesh, activated at 110øC for 24 hr) was packed into a column by use of methylene chloride, followed by addition of 50 g of bergamot oil. The column was eluted sequentially with 50 ml of methylene chloride and 50 ml of ethyl acetate. The ethyl acetate eluate was dried over anhydrous sodium sulfate, and the ethyl acetate removed on a rotary evaporator under vacuum. The residue was dissolved in methylene chloride, transferred to the Florisil column again, and then eluted with methylene chloride. The solvent was removed from a 100-200 ml fraction, the residue spotted on silica gel thin layer plate and, after development by use of a mixed solvent system of cyclohexane-tetrahydrofuran (1:1), bergapten was visualized either with UV light (yellow spot at the wavelength of 254 nm) or with iodine vapor. The bergapten zone (Rf around 0.4) was scraped with a spatula and bergapten eluted with acetone. The acetone was evaporated and the bergapten purified by recrystallization from methanol. The melting point of the bergapten is 188øC. Bergapten standard solution. The solution was prepared by dissolving sufficient bergapten in acetone to obtain concentrations of 100, 200, 300, 400 and 500/•g/ml. Chrysene standard solution. Chrysene (Tokyo Chemical Industry Co., Ltd.) was dissolved in sufficient acetone to obtain a final concentration of 300 •g/ml. Ten per cent methanolic potassium hydroxide. 100 g of potassium hydroxide was dissolved in 1,000 ml of methanol-water mixture (2:3). APPARATUS AND OPERATION CONDITIONS Gas chromatograph. Yanaco G-80 (Yanagimoto Mfg. Co., Ltd., Kyoto, Japan) was equipped with flame ionization detecting system (FID) glass tubes 3 mm in diameter and 150 cm in length were packed with 5% SE-30/Chromosorb W 60-80 column temperature, 230øC injection port temperature 280øC N 2 carrier with a gas flow rate of 30 ml/min. Gas chromatograph combined with mass spectrometer. Varian Aerograph GC, type 204 was combined with a Hitachi RMU-6E mass spectrometer. Glass tubes 3.2 mm in diameter and 150 cm in length were packed with 5% SE-30/Chromosorb W 60-80. Helium gas flow rate 30 ml/min. Chamber voltage, 70 eV, ion acceleration voltage, 25 kV. PROCEDURE An outline of the procedure is illustrated in Scheme 1. The procedure can be divided into three steps, i.e., Step 1--the unfolding and reclosing reaction of the lactone ring Step 2--the removal of acidic components and Step 3--the gas chromatographic determination. Step 1. To sample containing 10-1,000/.tg of bergapten, was added 5 ml of methanolic KOH and refluxed with stirring for 3 hr. After adding 40 ml of saturated sodium
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)