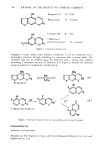
NITRITE ASSAY METHOD 387 If the sample did not fully dissolve, as in the case of TiO2, shaking was continued intermittently for a period of ! hr (sonication for about ! min was used to help dispersion and dissolution). Dilution to volume with distilled water was followed by thorough mixing and filtration through Whatman No. 42 filter paper. The first 5 ml of the tiltrate was rejected and the next 25 ml of the tiltrate was collected in a 25-ml volumetric flask having a head-space of at least 2 ml above the mark. The excess solution above the mark was withdrawn with a pasteur pipet. Procedure B A known amount of sample (approximately I g) was weighed into a glass-stoppered 125-ml edenmeyer flask, 20 ml of water saturated chloroform was added into the flask and shaken to effect complete or maximum dissolution (dispersion). The solution was then extracted with exactly 50 ml of water by shaking the mixture intermittently for 30 min. The aqueous layer was then clarified by centrifugation followed by filtration through Whatman No. 42 filter paper, etc., as described in Procedure A. COLOR DEVELOPMENT AND MEASUREMENT Aqueous extracts of samples from the above sample preparation procedures A and B in 25-ml volumetric flasks were treated in the same flasks as follows: A 0.5-ml aliquot of sulfanilamide solution was pipeted into the flask and mixed well. After allowing at least 2 min but not more than 8 min for the reaction to go to completion, 0.5 ml of N-(1-naphthyl) ethylenediamine dihydrochloride solution was pipeted into the flask and immediately mixed well. The reagent blank solution and nitrite standards were prepared in the same manner as the samples above using distilled water and nitrite solution totalling 25 ml in place of the aqueous sample extracts. Absorbance of the samples and standards were measured against the reagent blank solution at 543 nm using I cm cells, and the nitrite content of the samples were computed from comparative standard and sample absorbances. The absorbance measurements were made at about 30 min (acceptable range 10 min to 2 hr) after the addition of N-(1-naphthyl) ethylenediamine dihydrochloride solution. RESULTS AND DISCUSSION LINEARITY The linearity of response in the assay method in the range, 2 to 30 3tg nitrite nitrogen per liter was found to be excellent (correlation coefficient 1.000, intercept on absorbance axis -0.0012). Using either Procedure A or B for sample preparation, this linear range represented 0.1 to 1.0 mg/kg nitrite nitrogen in the sample. SAMPLE PREPARATION In order to determine nitrite by this colorimetric procedure, it is necessary for the nitrite to be present in aqueous solution. Since nitrite in raw materials of interest is present as an impurity, the selected sample preparation procedure should quantitatively extract or partition all nitrite into the water low nitrite assay values would otherwise result.
388 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Three approaches were considered for the sample preparation depending on the solubility characteristics of the samples (raw materials): 1. The sample is dissolved in water 2. The sample is dissolved in a solvent miscible with water (e.g. methanol). 3. The sample is dissolved in a solvent immiscible with water (e.g. chloroform) and extracted with water. The first approach is the most convenient and is applicable to all water-soluble and water-miscible samples (the desired solubility is 2 g/100 ml or better). The second approach is also simple as it involves simple dissolution in a chosen water-miscible solvent followed by dilution to volume with water. However it is necessary that the solvent does not interfere with the colorimetric determination. A study using methanol as the solvent showed that methanol interferes with the colorimetric determination (see Table I). Other solvents have not been investigated because this approach appeared to Table I Solvent Effects on the Absorbance in Colorimetric Nitrite Assay Method Nitrite nitrogen concentration 19.9/zg/1 Absorbance Solvent 543nm Water 0.0750 10% (v/v) Methanol in Water 0.0743 20% (v/v) Methanol in Water 0.0706 Chloroform saturated Water 0.0745 have limited applicability. Preliminary investigation of the third approach was done using chloroform as the solvent. Raw materials which were insoluble in water (e.g., stearic acid, glyceryl monostearate) were usually very soluble in chloroform. The solubility of chloroform in water is very small and the dissolved chloroform did not have any significant effect on the colorimetric determination of nitrite. This third approach has an advantage over the second approach because it permits extraction of nitrite into aqueous solution while leaving essentially all chloroform soluble raw material in the chloroform layer, thus reducing the possibility of interference in the later colorimetric determination. Using chloroform as the sample dissolution solvent (Approach 3, above) several raw materials (stearyl alcohol, glyceryl monostearate, cetyl alcohol, stearic acid) were analyzed by the colorimetric nitrite assay method. The aqueous extracts from the samples dissolved in chloroform were generally cloudy, but clarification of the extract was easily achieved by centrifugation. Addition of reagents to the clear extracts in the subsequent colorimetric determination produced noticeable turbidity. Background absorbances as high as 0.02 were observed at 650 nm, a wavelength at which the nitrite complex (azo-dye) does not absorb. The solutions were filtered through Millipore filters to minimize spectrophotometric errors due to the turbidity in the final solutions. Filtration through polyvinyl chloride filters (Millipore, Type BDWP, pore size 0.6/am) resulted in loss of the red azo-dye from the solution to the filters thus leading to lower nitrite assays. Similar loss was also observed using cellulose triacetate filter (Gelman Metricel Type GA-6, pore size 0.45 /am). The filter color absorption appeared to be variable and could not be reproduced
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)