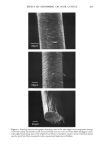
OCCLUSIVITY OF AQUEOUS EMULSIONS 167 corresponding to the isotropic oil phase are comparable to those obtained with the emulsions, there exists thus a direct correlation between the occlusive capacity of an O/W emulsion applied to the surface on the gelatin and that of the corresponding oily isotropic phase. For the emulsions studied, occlusivity was maximal for a given HLB value. The values of this HLB are depending on the nature of the oils and surfactants. However this optimal HLB corresponds to the ternary phase diagram in which the surface of the isotropic oil phase was the greatest. The extent of this phase in a diagram is linked to the facility of micelie formation. As described by Adrangui et al. (1979) there is an optimal HLB for each oil in which the organization of the two surfactants makes easier water miscibility (11). At this HLB, after evaporation of emulsion water, the formation of inverse miscelles is easiest. In these conditions isotropic oily phase forms easily on the gelatin. Isotropic oily phase presents a great resistivity because oil is the continuous phase producing occlusivity. For other HLB this phase appears with difficulty and occlusivity decreases. REFERENCES (1) I. H. Blank, Factors which influence the water content of the skin, J. Invest. Dermatol., 18, 433-440 (1952). (2) I. H. Blank, Further observations on factors which influence the water content of the stratum corneum, J. Invest. Dermatol., 21,259-269 (1953). (3) L. Gaul and G. B. Underwood, Relation of dew point and barometric pressure to chapping of normal skin, J. Invest. Dermatol., 19, 9-19 (1952). (4) G. Barnett, Emollient creams and lotions, in "Cosmeth Science and Technology" 2nd ed., M. S. Balsam and E. Sagafin, Eds. (Wiley-Interscience: New York, 1972), Vol. 2, pp 27-104. (5) J. B. Shelmire, The influence of oil-in-water emulsions on the hydration of keratin,J. Invest. Dermatol., 26, 105-108 (1956). (6) R. M. Handjani-Vila, B. Rondot and F. La Champt, Perspiratio insensibilis control by specific associations of lipids, Cosmet. Perfum., 90, 39-41 (1975). (7) R. M. Handjani-Vila, B. Rondot and F. La Champt, Measurement of the moisturizing effect, Cosmet. Toilet., 91, 25-30 (1976). (8) M. Adrangui, F. Puisieux, M. Seiller, E. Morszanyi, A.M. Orecchioni, Diagrammes eau-surfactif-huile i base de perhydrosqual•ne et de Miglyol 812 ©, Pharm. Acta. Helv., 54, 214-219 (1979). (9) D. Schwartz, M•thodes statistiques i l'usage des m•decins et des biologistes, Eds. Flammarion, Paris 1963 pp 181-183. (10) H. Tsutsumi, T. Utsugi and S. Hayashi, Study on the occlusivity of oil films,J. Soc. Cosmet. Chem., 30, 345-356 (1979). (11) I. Lo, F. Madsen, A. T. Florence,J. P. Tr&guier, M. Seiller and F. Puisieux, Mixed non-ionic detergent systems in aqueous and non-aqueous solvents, in "Micellization, solubilization and microemulsions," K. L. Mittal Eds., vol. 1 pp 455-466.
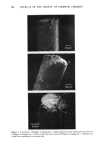
j. Soc. Cosmet. Chem., 33, 169-178 (July 1982) The mechanical spectra of skin, II. The thermal dependence of the low-strain viscoelastic properties RUSSELL O. POTTS and MIKLOS M. BREUER, Gillette Research Institute, 1413 Research Boulevard, Rockville, MD 20850. Received October 5, 1981. Synopsis We have investigated the low-strain viscoelastic properties of excised hamster skin in order to evaluate the underlying relaxation mechanisms. The stress relaxation in the physiologically important low-strain region was measured and the data transformed into a viscoelastic spectrum. Each spectrum represents H(r), the relaxation modulus as a function of r, the relaxation time constant. Both quantities are characteristic of relaxation mechanisms involved in the viscoelastic response. Relaxation spectra of excised hamster skin at various relative humidities and temperatures show, under all conditions tested, spectra consisting of three peaks (A, B and C) with time constants at 21øC and 95% r.h. near 2, 40 and 300 sec, respectively. For all three peaks r values show only temperature dependence, while the H(r) values vary only with relative humidity. The temperature dependence of r yields activation enthalpies (AH*) for the three peaks. The values of AH* for peaks B and C are in the range of 7-10 Kcal/mole, while peak A has a AH* value near zero within experimental error. On the basis of these results, the values of r and the variation of H(r) with relative humidity, we propose a molecular model for the mechanisms responsible for the mechanical relaxation. The model suggests that the stretching of elastin and subsequent shearing of the mucopolysaccharide-water gel are responsible for the low-strain viscoelastic response. INTRODUCTION Mammalian derreal tissue is highly complex in its function, composition and structure. It serves as the first line of defense against water loss and external shock, both biological and physical. In this last regard the skin is truly remarkable, repeatedly withstanding stresses of hundreds of pounds per square inch and yet remaining soft and pliant. Skin is a layered composite tissue primarily composed of fibrous proteins and gel-like mucopolysaccharides (1). The outermost layer of the epidermis, the stratum comeurn, comprises a relatively small portion of the intact skin and is the ultimate result of the differentiation and outward migration of underlying cells. It is primarily composed of the protein keratin which can exist in highly structured forms stabilized by hydrogen bonding (2). Beneath the epidermis lies the main body of the skin, the derreal tissue. The primary 169
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)