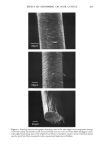
OCCLUSIVITY OF AQUEOUS EMULSIONS 163 zlO0 o • 90 • 80 7O o 6O o5o 4O 3O 20 10 r= 0,94 I ! ! I I ! 10 20 30 40 0 60 70 0 90 1•)0 %0 OF BINARY MIXTURES (oil_ surfactant) Figure 5. Correlation between the occlusivity of emulsions and that of binary mixtures oil/surfactant (Montane 80 © and Montanox 80©). DISCUSSION Once an emulsion is applied to the surface of the skin, its structure modifies with time because the water evaporates rapidly and only a residue containing principally the non-volatile materials persists (10). Comparable phenomena occur in our experimental conditions on the surface of the gelatin film. Immediately after application of the emulsion, the water starts to evaporate and coalescence of oil droplets occurred forming finally a continuous occlusive film. The water evaporation kinetics measured under the same conditions of temperature and RH on an inert support, showed that the totality of water was evaporated in 10-15 minutes according to the nature of the oil or surfactants used and HLB values of the emulsions. The residual quantity of water which persists in the mixture was approximately 5 to 10%. The qualitative composition of this residue is comparable to that of an isotropic oil phase. As the results of occlusivity measurements carried out on the mixture
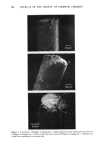
164 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ß ISOTROPIC OIL pNASE DIL ,ooz -.,., MIGLYOL 112 Figure 6. Importance of isotropic oil phase in the ternary diagrams water/oil/surfactants (Montane 80©-Montanox 80 ©) obtained with different HLB's. The zone corresponding to the oily isotropic phase is represented in black on each diagram. Its surface area is expressed as % in relation to the total surface of the diagram (data in parentheses). ß ISOTOOPIC OIL PHASE MINERAL OIL • % OIL loo • ,.,., MIGLYOL 812 Figure 7. Importance of isotropic oil phase in the ternary diagrams water/oil/surfactant (Simulsol 92©-Simulsol 96 ©) obtained to different HLB. Supplement to legend: see figure 6.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)