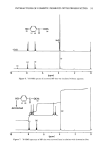
94 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 150 o • ?s 11•.5 SH ISF•IOO F t'- IP ,l t I I I I •VENUM•ER$ Figure 10. IR spectrum of MP that was recovered after incubation with ultramarine blue. IR SPECTRA OF RECOVERED MP Figure 9 shows the IR spectrum of MP that was recovered after incubation without pigment. Figure 10 shows the IR spectrum of the MP that was recovered after incuba- tion with ultramarine blue. Since both IR spectra are similar, the possibility of covalent bonding due to interaction with pigment was rejected. CONCENTRATIONS OF METAL IONS Using inductively coupled plasma emission spectrometry, measurement of the concen- trations of various metal ions found in incubation mixtures was done (Table VII). The Table VII Concentrations of the Metal Ions Found in the Incubated Mixtures (ppm) From From From MP supernatant supernatant solution of A of B Zn 0.02 0.02 0.05 Fe 0.03 0.03 0.15 Mg 0.04 0.05 0.20 Si ND 3.7 3.5 A1 ND 0.38 0.07 Na 5 11 45 Ca 0.16 0.20 1.5 A: ultramarine blue dispersion. B: ultramarine blue q- MP dispersion. ND: not detectable.
INTERACTIONS OF COSMETIC PIGMENTS WITH PRESERVATIVES 95 Table VIII Effect of the Replacement of Phenolic Hydrogen of MP, With Na and K Ions on Its Bactericidal Activity Phenolic Viable cell count hydrogen after 5-hr incubation MP Exist Below 10 3 MP, treated with Amberlyst-XN Na form Disappear 1.02 X 108 K form Disappear 1.49 X 108 Solutions of MP, untreated or treated with Amberlyst-XN, were inoculated with S. aureus, shaken, and incubated at 37øC for 5 hr. Aliquots were then decimally diluted with a 1% sterile solution of polysorbate 80. The number of surviving bacteria was determined by the viable cell count method. left column shows the metal ion concentrations found in the test tube where only MP was added. The central column shows data for the test tube in which only ultramarine blue was added. The right column shows data for the test tube where MP and ultra- marine blue were incubated together. It was found that the concentration of Na, Mg, and Fe ions increased unexpectedly when ultramarine blue was added to MP solution. It is possible that the MP might help dissolve these metal ions from ultramarine blue. REPLACEMENT OF PHENOLIC HYDROGEN OF MP AND BP WITH SODIUM AND POTASSIUM IONS When MP was eluted through Amberlyst XN-1004, the phenolic hydrogen of MP was replaced easily by Na or K ions, and its bactericidal activity drastically reduced (Table VIII). In the case of BP, however, exchange of its phenolic hydrogen did not occur, even though it was treated in the same way with the Amberlyste resin (Table IX). EFFECT OF EDTA ON THE BACTERICIDAL ACTIVITY OF MP Figure 11 shows the effect of EDTA on the bactericidal activity of MP in the presence of ultramarine blue. When 0.01% EDTA 3Na was added to the incubation mixture, it reduced the ID. These results suggest that the mechanism of preservative inactivation Table IX Effect of the Replacement of Phenolic Hydrogen of BP With Na and K Ions on Its Bactericidal Activity Phenolic Viable cell count hydrogen after 5-hr incubation BP Exist Below 10 3 BP, treated with Amberlyst-XN Na form Exist Below 10 3 K form Exist Below 10 3 Inoculum size: 2.54 X 108. Solutions of BP, treated or untreated with Amberlyst-XN, were inoculated with S. aureus, shaken, and incubated at 37øC for 5 hr. Aliquots were then decimally diluted with a 1% sterile solution ofpolysorbate 80. The number of surviving bacteria was determined by the viable cell count method.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)