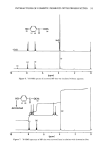
96 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 10 0 - o o i i I 10 '• 10 -1 1 EDTA.3Na (%) Figure 11. Effect of EDTA ß 3Na on the bactericidal activity of MP in the presence of ultramarine blue. EDTA ß 3Na was added to the incubation mixture, and this mixture was incubated at 37øC for 5 hr. Number of surviving bacteria was determined by the plate count method. by pigment involves replacement of phenolic hydrogen of MP by some metals from the coexistent pigment. THE MECHANISM OF PRESERVATIVE INACTIVATION BY PIGMENT The proposed mechanism of paraben inactivation by pigment follows. When ultra- marine blue is added to MP solution, the pH becomes alkaline, reaching pH 7.3. In the case of a powdered dispersion, it is assumed that there is the possibility of a difference between the pH of the solution and that of the powder surface. If this assumption is
INTERACTIONS OF COSMETIC PIGMENTS WITH PRESERVATIVES 97 T I I I 8 lO pH Figure 12. Dissociation curve for MP. Solutions of MP were titrated with 0.1 N ethanolic KOH. A model GT-01 (Mitubishi Kasei, Co.) automatic titrator was used in this study. accepted, then the pH on the surface of ultramarine blue in dispersion may be higher than 7.3. This high surface pH makes MP dissociate. In the case of a solution, MP's dissociation was observed above pH 8.3 as shown in Figure 12. Dissociations of EP, PP, and BP were observed at more alkaline pH than that of MP. As a result of the dissociation of MP, protons of MP are released and the pH begins to decrease. Conse- quently, with the decrease in pH, decomposition of ultramarine blue and talc are en- hanced (8) with the liberation of metallic ions, which makes the pH higher, balancing the system. On the other hand, kaolinite and silica alumina decompose less than ultra- marine blue and talc. The metallic ions liberated combine with MP, which is disso- ciated, forming MP salts having reduced bactericidal activity. It is believed that this cycle of pH change is involved in the mechanism of inactivation of MP by ultramarine blue. Mention should be made of the work of microbiologists who showed over 25 years ago that sorbic acid has more microbiological activity at pH 5 than at pH 6 (9). ACKNOWLEDGEMENTS The authors thank Mr. F. Suzuki and Mr. S. Takada for their helpful advice. We thank Mr. K. Komatsu for his help with •H and •3C-NMR spectrometry, and are also grateful to Mr. Y. Horri for his help with inductively coupled plasma emission spectrometry.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)