j. Soc. Cosmet. Chem., 42, 133-145 (May/June 1991) Inhibitor of odor-producing axillary bacterial exoenzymes A. CHARIG, C. FROEBE, A. SIMONE, and E. EIGEN, Colgate Palmolive Research Center, Piscataway, NJ 08854. Received October 1 O, 1990. Synopsis Zinc from aqueous zinc glycinate binds to hair and skin at pH 7. The binding increases with Zn concen- tration, becoming appreciable when Zn exceeds about 1 millimole/liter. In the presence of soap or synthetic detergents, Zn binding still occurs, but at a lower level. Zinc bound to hair acts as an effective inhibitor of two bacterial exoenzymes, aryl sulfatase and beta- glucuronidase, which are implicated in the production of steroidal axillary malodor (1). Clinical testing of the deodorant activity of this material has shown that a 4.5 % solution of Zn-GLY is as effective as 5 % aluminum chlorhydrate in controlling axillary odor. Although zinc glycinate is slightly bactericidal, enzyme inhibition seems to be the principal mechanism of deodorancy. The potential for a new underarm deodorant is discussed. INTRODUCTION Axillary apocrine secretions, as they emerge at the skin surface, are sterile and odorless odor arises from the action of bacteria upon sweat, and a group of steroids (androstenes) is found to contribute largely to it (2). Eigen (3) provided a mechanism for the gener- ation of axillary steroid malodor: the steroids are secreted as water-soluble non-volatile conjugates that are then cleaved to the volatile free steroid by esterases, such as aryl sulfatase (AS) and beta-glucuronidase (beta-G), which are secreted by axillary bacteria (Figure 1). In a previous paper, we reported on a series of studies that provided evidence to support this mechanism (1). One of the items of evidence supporting our theory was the fact that inhibitors of the enzymes also function as inhibitors of odor production in vitro. As part of that study, we tested many potential inhibitors of the enzymes and discovered several compounds that inhibit them. Such inhibitors, we felt, should function in the axilla to prevent the generation of odor, rather than to mask it, inhibit perspiration, or disturb the indig- enous microflora (2-4). A number of promising inhibitors were zinc salts, of limited solubility in water. We noticed that active Zn levels remained higher in some of the enzyme assays and hypothesized that the Tris buffer might be solubilizing zinc by chelation thus we tested zinc complexed with several ligands. Among their varying degrees of inhibition, zinc glycinate (Zn-GLY) was found to be effective as an inhibitor of both sulfatase and glucuronidase. It is also convenient to prepare and it is stable. We 133
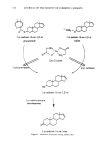
134 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS c% O 0" ' -- OSO2 • O• 5.ct-androst- 16-en-3,[•-ol glucuronide ,•-glucuro vNH2 O•c•.O • / C Zn o 9 •. ø / 'x J 5,ct-androst- 16-en-3,[•-ol sulfate Zinc Glycinate HO sulfatase 3,cz-androst- 16-en-3.•-ol 3,a-13ydroxystero•d clehyclrogenase 5,cz-androst- 16-en-3one Figure 1. Inhibition of enzymes causing axillary odor.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)