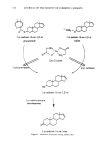
134 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS c% O 0" ' -- OSO2 • O• 5.ct-androst- 16-en-3,[•-ol glucuronide ,•-glucuro vNH2 O•c•.O • / C Zn o 9 •. ø / 'x J 5,ct-androst- 16-en-3,[•-ol sulfate Zinc Glycinate HO sulfatase 3,cz-androst- 16-en-3.•-ol 3,a-13ydroxystero•d clehyclrogenase 5,cz-androst- 16-en-3one Figure 1. Inhibition of enzymes causing axillary odor.
ODOR INHIBITION 135 then turned our attention to the feasibility of incorporating this material as an active agent into cleansing formulations. For an active agent to exhibit long-lasting deodorancy, it must bind to the skin and/or hair, and the bound residue must remain inhibitory in the presence of soaps, etc. This paper describes our discovery that zinc glycinate is in fact substantive to skin and hair even in the presence of a soap or synthetic detergent (syndet) solution. Once bound, and in the presence of these solutions, its anti-enzyme activity is preserved. Thus Zn-GLY may serve as an effective deodorant. METHODS MATERIALS Enzymes, substrates, and buffer components were purchased from Sigma Chemical Co. All experiments were carried out in 0.1 M Tris buffer at pH 7. Non-isotopic Zn-GLY was prepared as described by Dubsky and Rabas (6). [65Zn] Zn-GLY was prepared as needed for each experiment. A convenient amount of 65Zn, in solution of negligible volume and containing negligible mass of Zn, was added to a solution of normal ZnCI 2 at a concentration twice what was wanted for the experiment. To it was added an equal volume of solution containing a two-fold molar excess of glycine sodium salt, so the final Zn concentration was as required. The mixture had a pH of 9. The precipitate (ZnO), which formed when glycine was first added, gradually redissolved as the complex formed, and the final solution contained the required con- centration of Zn in solution as the diglycinate at a pH of approximately 8. INSTRUMENTATION Radioisotope was detected by counting in a Baird Atomic Model 530 Gamma Spec- trometer with multichannel analyzer. Fluorescence intensities were measured with a Perkin-Elmer Model MPF-3 with an Osram XBO high-pressure Xenon lamp, using quartz cuvettes. Absorption wavelength for 4 methylumbelliferol was 334 mm emission wavelength was 444 mm. PRELIMINARY STUDY: KINETICS OF BINDING OF Zn-GLY TO HAIR Before undertaking quantitative substantivity studies, it was necessary to establish the time scale of binding of Zn to the substrate. Solutions of [65Zn] Zn-GLY were prepared as above, with Zn at 0.01 raM, 1 m M, and 10 raM hair samples (0.1 g each) were exposed to 2.5 ml of solution for various lengths of time. The solution was decanted, the hair rinsed twice with 0.5 ml water, and the rinses combined with the exposure solutions. Radioactivity in the solution ("unbound isotope") and in hair ("bound isotope") was counted. The amount of Zn bound/g hair and the percent of radioactivity ultimately bound to hair was calculated from the total Zn in the sample solutions. SUBSTANTIVITY OF Zn-GLY TO HAIR: EFFECTS OF GLYCINE Two series of solutions were prepared with various levels of Zn in both (Figure 3) one series had excess glycine present, the other had none. Two ml of each solution was
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)