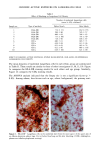
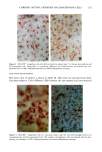
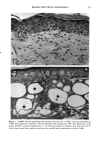
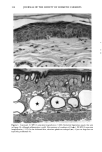
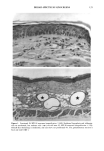
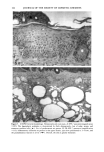
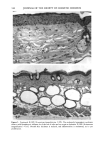
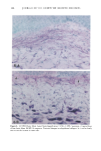
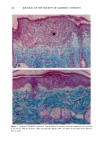
PHOTOTOXICITY TESTING 171 Table I Cell Survival (%) After Irradiation With the "Bluelight 2000" Apparatus in the Presence of Test Substances as Compared to Cell Survival in the Presence of Solvent Alone and After Irradiation with Mylar Filtered UV Light Irradiation with "Bluelight 2000" (min) Test substance (concentration) 0 1 2 4 8 16 32 Solvent (DMSO) and mylar- filtered UVA only 100 100 100 88 82 60 42 Glibenclamide (0.5 mmol/1) 100 100 74 63 0 0 0 Gliquidone (0.5 mmol/1) 100 100 77 63 0 0 0 Bemetizide (0.5 mmol/1) 100 53 49 11 0 0 0 Bemetizide (0.25 mmol/1) 100 53 49 11 0 0 0 Bendroflumethiazide (0.5 mmol/1) 100 58 29 0 0 0 0 Bendroflumethiazide (0.1 mmol/1) 100 96 63 12 0 0 0 Bendroflumethiazide (0.05 mmol/l) 100 74 88 61 35 0 Benzylhydrochlorothiazide (0.5 mmol/l) 100 67 56 28 0 0 0 Benzylhydrochlorothiazide (0.25 mmol/1) 100 67 56 28 0 0 0 Bumetanide (0.5 mmol/1) 100 100 75 56 19 0 0 Bumetanide (0.25 mmol/1) 100 96 82 60 53 0 0 Butizide (0.5 mmol/1) 100 100 42 21 0 0 0 Hydrochlorothiazide (0.5 mmol/1) 100 79 32 28 0 0 0 Hydroflumethiazide (0.5 mmol/1) 100 91 47 46 35 0 0 Hydroflumethiazide (0.25 mmol/l) 100 91 47 46 35 0 0 Piretanide (0.5 mmol/1) 100 100 67 70 12 0 0 Polythiazide (0.5 mmol/l) 100 51 19 9 0 0 0 Trichlormethiazide (0.5 mmol/1) 100 100 79 58 30 0 0 became more prominent when the irradiation time/dose exceeded 8 min/9.6 J/cm 2 UVA. No significant results were obtained with the addition of beta-carotene or ubiqui- none. When investigating the inhibiting effects of the antioxidants with the UVA tubes, no significant differences between the results with and without addition of antioxidants were recognized. The electronmicroscopical investigations showed destruc- tion of biomembranes, but nuclear damage was demonstrable as well. DISCUSSION Two out of seven oral antidiabetics and ten out of fourteen diuretics exhibited phototoxic action in our model. The absorption maxima of the test substances is in the UVA range at about 325 nm, which overlaps well with the emission maxima to the "Bluelight 2000" lamp. Not all potential photosensitizers revealed positive results in this model. This may be due to weak sensitizing capacity, but factors such as binding to, or penetration of, the
172 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cellular membrane have to be evaluated. Another explanation for differences in photo- toxic response may be explained by the molecular structure of the substances. When comparing the phototoxic with the nonphototoxic drugs, it is noticeable that the phototoxic drugs all have either long lipophilic substitutes in the molecules (such as in glibenclamide, Figure 3a) or a double-ring structure (such as in bendroflumethiazide, Figure 3b). The nonphototoxic drugs have shorter lipophilic substitutes (such as in chlorpropamide, Figure 4a) or a one-ring molecular structure, so-called monosulpho- namides (such as in furosemide, Figure 4b). The more lipohilic substances may therefore induce phototoxicity more easily than the lesser lipophilic. Different subcellular targets also have to be considered. In the photohemolysis test, membrane damage is detected, whereas in more complex systems, DNA damage may play an important role as well. This is indicated in our electronmicroscopic investigations, where nuclear damage is demonstrable. This is in accordance with earlier findings showing thiazides to exert their main phototoxicity on biomembranes (15), but photomutagenic effects have also been reported (16). Photosensitizing substances seem to have one major target, but may induce damage to other sites as well (17). Matsuo and co-workers clearly demonstrated that the membrane lipids play an impor- tant role in thiazide phototoxicity (15). They irradiated red cells with longwave UV in the presence of the thiazide diuretics, and showed that the amount of peroxide was enhanced in deuterium and inhibited by antioxidants, indicating the involvement of oxygen radicals. The phototoxic properties due to our test substances were, in the same manner, clearly dependent on oxygen, as antioxidants, especially ascorbic acid, significantly inhibited the phototoxic action (18). The phototoxic properties of the test substances were only observed when test substances and cells were incubated together. Preirradiation of the test substances and subsequent incubation with the erythrocytes did not induce hemol- ysis. This may be explained by the involvement of oxygen radicals, which have a limited lifetime and diffusion length in cells (19). So far, two oral antidiabetics and eight diuretics have been investigated in vitro for phototoxicity (Table II). Johnson and co-workers demonstrated the photohemolytic effects of tolbutamide, but were unable to detect phototoxic properties in the presence I-I3OO 0=(2 Figure 3a. Molecular structure of glibenclamide. Figure 3b. Molecular structure of bendroflumethiazide.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)