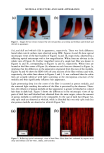
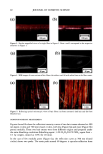
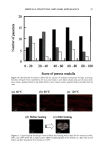
OXIDATIVE HAIR DYES 109 2000 1500 - 1000- 500 - . -200 5OO 300- - o -200 I i 0.0 7.5 15.0 0 3 5 22.5 35 Retention time (min) M ( i i i 0.0 7.5 15.0 22.5 35.0 Retention time (min) Figure 1. Chromatographic separations of two commercial formulations after matrix extraction. a: "dark brown" shade cream, DBRC. b: "light blonde" shade shampoo, LBLS. Peaks: (0) NaAsc (intrinsic matrix, antioxidant) (1)p-phenylenediamine (3)p-aminophenol (4) m-aminophenol (5) toluene-2,5-diamine sul- fate (6) 2,4-diaminophenoxyethanol (7) resorcinol (8) 0-aminophenol (1 l) 6-hydroxyindole (12) 2-meth- yl-5-hydroxyethylaminophenol (15) hydroxybenzomorpholine or matrix compound (16) matrix compound or hydroxybenzomorpholine and (M) matrix compound. See text for experimental data and acronyms. dark blonde shade shampoo (DBLS), or for 2,4-diaminophenoxyethanol and 0- aminophenol, coeluted in the dark brown shade cream (DBRC). First, a study of the influence of temperature on the separation was performed. The results obtained for one of the tested standard mixtures containing m-aminophenol, 2,4-diaminophenoxyethanol, toluene-2,5-diamine sulfate, and resorcinol, for example, are shown in Figure 2a. It comes out that the two coeluted hair dye intermediates, 2,4-diaminophenoxyethanol and toluene-2,5-diamine sulfate, can be separated when the temperature is increasing (with an optimum at 60øC) and identified by means of the DAD when present at the same concentration in the mixture (peaks 5 and 6 in Figure
110 JOURNAL OF COSMETIC SCIENCE +1 % +1 +1 +1 % +1 % +1 +1 ,• t"q +1 % % +1 +1 +1 +1 % % % +1 t/• c• c• OO t/• OO +1 % % +1 +1 +1 +l % % % +l +1 % +1 % +1 +1 +1 % * +1 % % +1 +1 +1 +1 % % • +1
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)