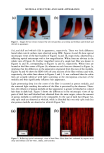
130 JOURNAL OF COSMETIC SCIENCE FOAM PROPERTIES OF SHAMPOOS AND THEIR EFFECTS ON SOIL REMOVAL AND INSOLUBLE ACTIVE DEPOSITION Manuel Gamez-Garcia, Ph.D. Amerchol Technical Center, 136 Talmadge Road, Edison, NJ INTRODUCTION Understanding the process of soil removal and insoluble active delivery from anionic detergent compositions is crucial in formulating efficient shampoos. The dynamics and complexity involved in a shampooing process makes, however, difficult the accomplishment of this task. It is well known that during hair washing a shampoo undergoes various phase transformations. For instance, at the point of application the shampoo is a colloidal liquid that may contain coacervates or precipitates because of its primary dilution. Then, during the lathering step this colloidal liquid is converted into foam. Finally, during the rinsing step the foam is transformed into a complex mixture of foam and gas emulsion. These phase changes during the shampooing process do not occur in definite steps, but rather in a gradual manner with the phases tending to overlap in time. For instance during shampoo application, the initial shampoo composition is simultaneously diluted and sheared to produce foam. Subsequently, during rinsing only a portion of the foam reaches dilution conditions the rest of the foam remains undiluted while a great portion of it is removed mechanically by the water stream. This paper presents data which shows that coacervates formed during the foaming process persist and partition within the foam between the lainella walls ("crust") and the lamella liquid ("core") of the foam. Coacervate dispersability in the foam "core" of model shampoos was found to change with water dilution ratio. As the foam dilution ratio increased the coacervates in the "core" were seen to change from dispersible, to non-dispersible, and then to dispersible again at high dilutions. It was also found that depending on the polycation molecular weight, these changes in coacervate behavior, produced either insoluble droplet stabilization or hetero-flocculation. Droplet stabilization by coacervates was found to inhibit insoluble deposition on hair surfaces, while droplet hetero-flocculation by coacervates enhanced insoluble deposition. METHODOLOGY Deposition of silicon on hair was carried out by X-Ray Fluorescence using the method of Gruber et al (1). Identification and quantification of coacervates was carried out by Optical Microscopy and Centrifugation. Measurements of polymer substantivity to hair were performed using the quantitative dye technique described by Jones et al (2). Hair tresses, 2 inches wide and 8 inches long with 2.5 grams constructed using European Medium Brown hair from IHI (Valhalla, NY) were used in the quantitative analysis. RESULTS When 10 grams of two model shampoos with composition SLES-2/JR-30M/CAPB and SLES-2/JR-30M/DCDAC, respectively, were diluted in a 1-7 ratio they gave rise to the formation of hazy solutions indicating phase separation and formation of coacervates.
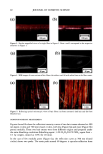
2001 ANNUAL SCIENTIFIC MEETING 131 Coacervate identification was made by separating the hazy material after centrifugation during 1 hour at 3,000 rpm. At the end of the centrifugation process the dispersed coacervates separated into a clear and transparent gel layer at the bottom of the centrifugation tube. Fig. 1 shows a typical microscopic image of coacervates dispersed in the shampoo dilution before centrifugation. In order to analyze the behavior of coacervates in foams, the same hazy shampoo dilution was stirred in a beaker until a dense foam was produced. A microscopic image of the foam surface showing the presence of coacervates dispersed in the inter-lamellar liquid of the foam can be seen in Fig. 2. This observation clearly indicates that coacervates formed during shampoo dilution persist during the foaming process. Further dilution foam analysis of model shampoos indicated that coacervates partition between the lamella walls ("crust") and lamellar liquid ("core") of the foam (3). For instance, when foams made of shampoos at different dilutions were poured into a separatory funnel and allowed to stand 5 minutes, the foam separated into two main components, namely: a drained liquid at the bottom of the funnel and a dry foam at the top of it. The drained liquid of foams made with shampoos at low dilutions was found to be hazy and to contain dispersed coacervates. It was also seen that as the foam dilution ratio increased the drained liquid become clear and transparent. At higher foam dilutions the drained liquid turned hazy again. These observations indicate that coacervates partitioned into the liquid "core" of the foam lamella change from dispersible to non- dispersible, and then again to dispersible with the dilution ratio. The interaction of silicone droplets with coacervates was affected by the observed variations in coacervate dispersability. For instance, at low shampoo dilutions when the coacervates were dispersible, coacervate and silicone droplets behaved as independent dispersible entities (see Fig. 3). This observation indicates that at low dilutions the interaction between coacervates and silicone droplets is practically non-existent. In contrast, at dilution regions where the coacervates became non-dispersible, it was found that silicone droplets and coacervates flocculated forming complex aggregates (see Fig. 4). At higher dilutions the silicone/coacervate flocs became weaker and started to separate. Fig. 1) Microscopic image of 1 to 7 dilution of shampoo 15 % SLES-2/0.5 % JR-30M/3% CAPB showing coacervate gel particles dispersed in shampoo solution 37X
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)