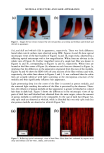
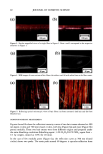
132 JOURNAL OF COSMETIC SCIENCE Fig. 2) Microscopic image of foam surface made with shampoo 15 % SLES-2 0.5 % JR-30M/3.0 % CAPB showing coacervates in lamella foam (dilution I to 7) I Coacervates Fig. 3 Microscopic image of silicone droplets and coacervates showing in&pendent dispersion Shampoo: 15 % SLES/0.5 % JR-30M 3.0 % CAPB Silicone oil GE SF-96-50 (dilution I to 3) 37X Fig. 4) Microscopic image of silicone droplets and coacervates showing flocculation and formation of aggregates. (Shampoo: 15 % SLES/0.5 % JR-30M 3.0 % CAPB Silicone oil GE SF-96-50) (dilution I to 7) 37X REFERENCES 1) J.V. Gruber, B.R. Lamoreaux, N. Joshi, L.Moral, "Influence of Cationic Polysacchafides on Polydimethylsilixane Deposition onto Keratin Surfaces from a Surfactant Emulsified System", pp. 127-135, Col. and Surf. B: Biointerfaces, 19 (2000) 2) R.T. Jones, and C.A. Brown, "The Behavior of Cationic Cellulose Derivatives Containing Fatty Quat Groups", Int. J. of Cosm. Chem., 38, pp. 233-246 (1987)
2001 ANNUAL SCIENTIFIC MEETING 133 THE EFFECTS OF HYDRATED SALTS ON THE THERMAL STABILITY OF OXIDATIVE HAIR DECOLORIZING COMPOSITIONS Walter B. Shepherd, Jr. C.C.P., Inc., 25Andrews Drive, West Paterson, NJ 07424 Introduction Oxidative hair decolorizing compositions based on persulfates were studied to determine the degree to which added water and water from the addition of hydrated salts affect the thermal stability of these compositions. The data from this study have lead to the development of a procedure for the comparative evaluation of the thermal stability of persulfate based hair decolorizing compositions. Experimental The apparatus shown in fig. 1 was assembled using dry bath incubators with aluminum heating blocks containing tube cavities of varying diameters. Test cells were constructed from borosilicate glass tubes of varying diameters fitted with type T thermocouple probes held in place by polyethylene centering discs and a polyethylene tube closure. Aluminum heating block and test cell temperature data were collected at 2 - 5 second intervals using a computerized data logging system. Test samples were prepared by blending a fueled persulfate mixture consisting of sodium, ammonium or potassium persulfate and micropulverized cellulose at a fixed ratio with a salt selected from the group consisting of: sodium silicate - hydrated, sodium silicate - anhydrous, sodium carbonate - anhydrous, sodium carbonate - hydrated, sodium metasilicate - anhydrous, sodium metasilicate - pentahydrate and magnesium carbonate. Additionally, test samples were prepared using anhydrous salt with free water equivalent to the water ofhydration of the corresponding hydrated salt added separately and mechanically blended into the mixture. The test samples were subjected to both isothermal and ramped heating profiles to determine the effects on the critical temperature, Tc, of the mixtures as noted by the thermal decomposition of the samples. Simultaneously, the active oxygen content of the mixtures was measured at selected intervals to determine the rate of decomposition of the persulfates as the Tc is approached. Prototype hair bleach compositions were prepared using various combinations ofpersulfates and hydrated or anhydrous salts and subjected to the identical experimental conditions as the test samples. Results and Discussion The use of hydrated salts in fueled oxidizer systems decreases the critical temperature, Tc, of the mixture. The magnitude of the effect is directly related to a number of chemical and physical properties of the mixture. It was determined that the Tc decreases dramatically with the increase in the total water content of the mixture. This result is observed for mixtures based on sodium, ammonium or potassium persulfate and the magnitude of the decrease in the Tc was such that: sodium ammonium potassium. The decrease in the Tc with increased total water content was observed for samples in which the water in the mixture was added as free water and mechanically blended in as well as for samples where the water was present in the form of water of hydration. It was noted that the decrease in the Tc was greater when the water was introduced as free water. The magnitude of the decrease in the Tc with increasing water content
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)