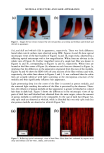
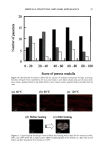
148 JOURNAL OF COSMETIC SCIENCE | Octanal Nonanal Decanal / , ...... / ,..," ----" / Chromato•ram of Volatiles From Skin .............. .... ,,_ ,I .... •G• .... • ' • ........ •o •.• ........ ::2 •.•...•..•..•.• ...... .•o ,...•,,..5.. 5•...• ............ .• .• ..•. ............... ..• .• ....... :..:•'o..... .....•......• •....L •..,.• •.?...•....5..•.. ,• ..•., .• • x ß o No,anl Peak: Mass Spectrum (Top) an Libra• Match (Botton Fit = 90 Additionally, we obse•ed that some panelists e•ibited ce•ain specific volatile compounds including: - Ketone: 6-Methyl-5-Hepten-2-one - Residuals of Fragrances and Cosmetic Products: Lmalool, Dihydromethyl Jasmonate, Ionones, Isopropyl M•istate, and several •es of Glycols. - Hydrocarbons of sho•er chain lengths: i.e. Decane. All of the compounds that were found could react with cosmetic products that are applied to the human skin. The presence of these different compounds could affect and initiate differences in the odor perceived. To confi• how products react with volatile compounds we used a cosmetic product that usually produces odor. After application of the cosmetic product we found differences in the relative odor perceived. We found that the differences in odor could be codected with ce•ain na•ral volatiles from human skin: ß Stronger odors were detected in panelists that generally have the ketone, higher levels of aldehydes, and branched hydrocarbons. ß While lower odors were detected in panelists that generally have glycol compounds and some fragrance residuals, and did not e•ibit the ketone. In general, some of the identified compounds are evolving from the skin, and can be considered endogenous, while the silicones and fragrance ingredients seem to be residuals from consumer products. The relative amounts and varieW of na•ral components found on the skin were obse•ed to change with time and were affected by the use of cosmetic products. Conclusions: The novel method is a practical and valuable tec•ique for the s•dy and identification of volatile compounds from human skin in vivo. It also has many potential applications including: studying the effects of fragrance compounds, crea•, and lotions on the skin the analysis ofpheromones and of biochemical changes in human scent, etc. We deterned and identified co•on and specific volatfie compounds from human skin m-vivo. Analyzing untreated skin, we recognized and established differences and si•larities in endogenous compounds that are liberated from human skin. The compounds were classified according to their che•cal propedies. Also we identified volatile compounds that can con•ibute to differences in u•que odor fo•ation after application of cosmetic products. The results from our investigation can be used as a basis to predict the perromance of new cosmetic products on the human skin. The work presented is a precursor to more in-depth research in the field of smell and odor from human skin. in vivo and in vitro.
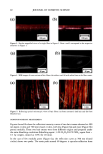
2001 ANNUAL SCIENTIFIC MEETING 149 CLINICAL EFFECTS OF EMOLLIENTS ON SKIN Joachim W. Fluhr, M.D. Department of Dermatology, VA Medical Center and University of California, San Francisco, CA Introduction Recent studies have shown that the use of an appropriate emollient for the treatment of spectfic skin disorders can have a significant impact on both the clinical outcome of treatment, and the extent of the subsequent relapse-free period. Emollients should not be regarded simply as a drug career or delivery system, but rather as an essential component of successful topical treatment. Specific effects ofemolhents Emollients exert hydrating, cooling and barrier repair effects on the skin, They influence the stratum comeum (S,C,) hydration, for which different mechanisms have been proposed: - The emollient exert a direct hydrating effect by liberating water from the formulation itself [1 ]. This effect of O/W-systems depends primarily on the water content of the formulation, with unbound water insuring water insuring the S.C. hydration [2]. In long-term applications of W/O but not of O/W emulsions revealed hydration of the S.C. [3]. - The occlusive effect of the formulation can inflt•ence S.C. hydration. especially in long-term applications e.g, petrolatum, for which the highest occlusive effect was detected [4]. W/O emulsions with low water content have occlusive effects similar to petrolatum, while W/O emulsions with high water content have occlusive properties similar to O/W emulsions [4]. Even O/W formulations with high water content exert an occlusion effect after the unbound water evaporates. In atopic dermatitis, an occlusive effect may enhance discomfort and induce itching response(s). The occlusive effects also may enhance drug penetration. - Highly hygroscopic compounds like glycerol, by absorbing water either from the emollient itself, from surface water, or from water of evaporation, then are able to increase S.C. hydration [5]. Recent studies in asebia mice could show the importance of sebaceous gland derived glycerol for S.C. hydration (unpublished data) (Fig. 1). - The formation of lipid bilayers from secreted lameliar bodies at the Stratum granulosungS.C. interface traps hygroscopic materials in the Comeocyte cytosol, with the comeOCyte lipid envelope acting like a 'dialysis membrane'. The cooling-effect of emollients can be attributed to the amount of evaporated water and/or alcohol in the emulsion, e.g, with lotions, hydrogels or O/W emulsions. However. relatively non-stable W/O emulsions, like cold creams, also can exert mild cooling-effect(s). Role of ph¾sioloqical lipids in emollient formulations The barrier function of the skin is mediated by intercellular bilayers in the S.C.. Cholesterol (25%), ceramides (40%), free fatty acids (20%) are key lipids in the formation of these bilayers [6]. These lipid classes have an approximately equimolar physiologic ratio. Following barrier disruption, epidermal cholesterol and fatty acid syntheses are immediately increased, while increased ceramide production is evident about 6 hours later [6-9]. The key lipids are delivered to the intercellular space of the S.C, as a mixture of precursors by the extrusion of lameIlar body content at the stratum granulosum - S.C. interface. Fusion of the secreted lameliar contents within the lower S.C. leading to continuous membrane sheets, ultimately form mature membrane bilayer structures. These membrane structural transformation correlates with changes in lipid composition: the polar lipid precursors are metabolized to more- nonpolar lipid products [6]. Topical applied physiologic lipids have distinct effects from those of nonphysiologic lipids, like petrolatum. Studies have shown that topical application of only one or two of the three physiologic lipids to disrupted skin impedes rather than facilitates barrier recovery [7]. If members of all key lipids are apphed together to barrier-disrupted skin, normalized rates of barrier repair are observed [10]. Further enhancement of barrier recovery is observed if the proportion of one of the fatty ac:ds (linoleic acid, palmitic acid or stearic acid) or either of the two other key species is augmented to three-fold i.e. consisting of fatty acid, ceramide, cholesterol, essential fatty acids in a 3:1:1:1 ratio [11]. Topically applied physiologic lipids not only are concentrated in the S.C. membrane domains, but also are delivered to the nucleated layers of the epidermis [7, 10] (Fig. 2). Depending on the composition of the lipid mixture, either normal or abnormal lameIlar bodies are formed, resulting in either normal or ab,c, rmal lameliar membrane umt structures in the S.C. intercellular spaces. The incorporation of applied physiolo•oic lipids into barrier lipids follows two pathways: 1) direct incorporation into S.C. membrane domains 2) lip•ds appear to traverse the intercellular route in the S.C., and ultimately get incorporated into lower stratum granulosum cells. The intercellular ilplds then appear able to enter the nucleated cells, incorporate into the appropriate lipid metabohc pathway(s), and ultimately utilize the lameliar body delivery system to reenter the intercellular membrane domains [10]. These studies support the hypothesis that the epidermis can internalize and process physiologic lipids..In contrast, nonphysiologic lipids like petrolatum appear to simply form a bulk hydrophobic phase in the S.C. intercelltfiar spaces to restore the barrier under similar condition [10]. Figure 1: Proposed sequence in Asebia-J skin: Decreased sebaceous gland-derived glycerol results in reduced SC hydration. Figure 2: Topically aoolied ohvsioloeic li•ids in lhe S.C. membrane and lhe nuclealed layers Lameliar body formallon in the granular cell. Topically applied physiologic lipiris enler lhe granular cell and reach sites of lameliar body formation (wilh permission from Dr. P.M, Elias)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)