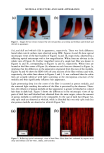
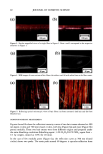
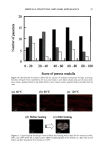
146 JOURNAL OF COSMETIC SCIENCE The disparity in distribution patterns of melanosomes is one factor that gives differences in skin pigmentation and in photoprotection. The control of these innate distribution patterns of melanosomes is poorly understood. To investigate this process, cocultures ofmelanocytes and keratinocytes from different racial backgrounds were examined using electron microscopy. Melanosomes in keratinocytes were counted and categorized as individual or in clusters of 2-3, 4-6, or greater than 6. In addition, melanosomal area wa•, determined for individually versus clustered melanosomes to correlate with the different distribution patterns observed. Results indicate that in our model system, melanosomes in keratinocytes from different racial backgrounds have a combination of clustered and individual melanosomes. When dark skin derived keratinocytes were cocultured with melanocytes derived from (a) dark or (b) light skin, recipient melanosomes were individual versus clustered in (a) 77.6% versus 22.4% and (b) 63.5% versus 36.5%, respectively. In contrast, when light skin derived keratinocytes were cocultured with melanocytes derived from (c) dark or (d) light skin, recipient melanosomes were individual versus clustered in (c) 33.5% versus 66.5% and (d) 38.7% and 61.3%, respectively. These results indicate that regardless of the donor melanocyte, recipient melanosomes will be distributed by keratinocytes from dark skin predominantly individually, and from light skin predominantly in membrane-bound clusters. One factor that appears to regulate the distribution patterns of melanosomes is their size. Studies regarding this are in dispute. Therefore, we assessed our cocultures for the pattern ofmelanosome distribution within keratinocytes and in turn correlated them with cell donor type and melanosome size. We found that although there were differences in melanosome size from dark or light donor melanocytes, there were no dift•rences in the sizes of melanosomes distributed individually compared to those clustered. This is demonstrated in Table 1. Table I Sizes of Melanosomes Donated either from Black of Caucasian Melanocytes within Keratinocytes. Sizes of Individually Distributed Melanosomes can be Compared with Sizes of Clustered Melanosomes. ire Average Melanosome Size (F.m 2) )m Melanosomes tual Melanosomes red Melanosomes •s + Caucasian ( 102ñ8 14X 10 • X 102+_6.71 X 10 • X 10•_+6 66 X 10 • __ __ locytes + Caucasian X 10-• ñ 2.89 X 10 '• X 10'2_+2 91 X 10 -• X 10'•_+ 4 06X 10 '• These results suggest that the size of the recipient melanosome does not appear to regulate how melanosomes will be distributed but instead some regulatory factor within the keratinocyte coordinates this process.
2001 ANNUAL SCIENTIFIC MEETING 147 STUDY AND IDENTIFICATION OF VOLATILE COMPOUNDS FROM HUMAN SKIN Asira Ostrovskaya, Peter A. Landa, Marina Sokolinsky, Anthony D. Rosalia and Daniel Maes Estee Lauder Inc., Research and Development, 125 Pinelawn Road, Melville, NY 11747 Introduction: Human skin is an extremely complicated biochemical system. Each individual has a unique skin chemistry and accordingly a personal smell that is as characteristic as human fingerprints. When the same perfumes or fragrance products are applied to individuals, differences in odor have been observed. The goal of our investigation was to recognize and identify volatile compounds responsible for the unique odor from human skin. A novel analytical technique which combines Solid Phase Micro Extraction (SPME) with subsequent analysis via Gas Chromatography - Mass Spectroscopy (GC-MS) was developed and successfully applied. Method: The SPME (Supelco Inc.) technique used consists of fused silica fibers coated with Divinylbenzene / Carboxen on polydimethylsiloxane, 50/30urn. These fibers are used to absorb and concentrate analytes in the headspace above the skin. The volatile compounds are then transferred into the GC-MS where they are thermally desorbed and analyzed. Sampling: A glass sampling device, developed for this study, with a 6cm diameter opening was placed and secured over the skin. The SPME fiber is introduced into the top of the sampling device through a septurn. The fiber is then exposed to the headspace for 45 minutes. The Fibers are then desorbed in the heated injection port of the Gas Chromatograph. Analytical Conditions: GC Conditions: Columns: non-polar DB-I and polar DB-Wax lnjector= 270øC for DB-I 250øC for DB-Wax Transfer Line = 270 øC for DB-1 250øC for DB-Wax Flow = 1 mL/min Split = 100/1 mL/min (split valve closed during desorption) Oven Temp. = 40 øC (2rain), 4 øC/rain, to 200 øC (3rain) MS Conditions: MS Scan: 35 - 400AMU @ 2.0scans/sec. The volatile compounds are analyzed and identified by interpreting their Mass Spectra and by performing searches to known Mass Spectral libraries. Protocol: 50 female panelists were recruited for this study. All panelists were healthy and between 18 and 60 years of age. Prior to the beginning of the study panelists were instructed not to use any fragranced products or aromatic foods throughout the entire study. The untreated volar forearms of the panelists were used as the test sites. The skin was cleaned with water and dried prior to testing. Results and Discussion: Several classes of compounds were found and identified in the headspace from human skin and they include: shorter and longer chain hydrocarbons, short chain aldehydes, a branched ketone, silicones, and residual components of fragrances and cosmetics. ß On 88% of the panelists - Short chain aldehydes were found such as: Nonanal, Octanal, and Decanal. ß On 96% of the panelists - Hydrocarbons of longer chain lengths were found such as: Tetradecane, Pentadecane, and Hexadecane. ß The relative levels of these common compounds, however, differed between individuals.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)